PHYSICS
WITH RELIGION
Physics
:
What is an atom and what are atoms made of ?
Atom
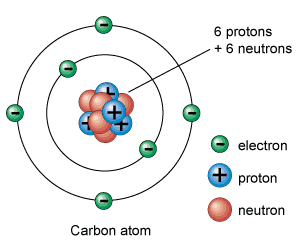
Atoms
are the basic building blocks of ordinary matter. Atoms can join
together to form molecules, which in turn form most of the objects
around you.
Atoms are composed of particles called protons, electrons and neutrons.
Protons :

Protons carry a positive electrical charge.
Electrons :

Electrons carry a negative electrical charge.
Neutrons :

Neutrons
carry no electrical charge at all.
The protons and neutrons cluster together in the central part of
the atom, called the nucleus, and the electrons 'orbit' the nucleus.
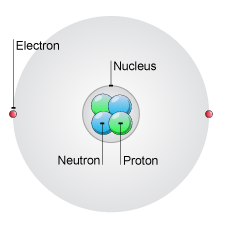
Atom
A particular atom will have the same number of protons and electrons
and most atoms have at least as many neutrons as protons.
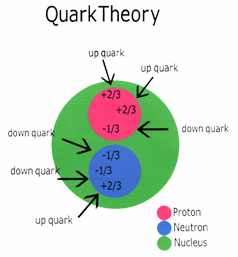
Protons and neutrons are both composed of other particles called
quarks and gluons. Protons contain
two 'up' (+) quarks and one 'down' (-) quark while neutrons contain
one 'up' (+) quark and two 'down' (-) quarks.
The gluons are responsible for binding the quarks to one another.
Atom
Each family contains two quarks. The first family consists of Up
and Down quarks, the quarks that join together to form protons and
neutrons. The second family consists of Strange and Charm quarks
and only exist at high energies. The third family consists of Top
and Bottom quarks and only exist at very high energies.
Physics
with Religion :
Nucleus (vishnu / preserver) :
The nucleus is composed of positively charged protons and neutrally
charged neutrons.
Proton
(shiv / destroyer) :
Protons also play a significant role because the tendency for an
atom to either lose, gain or share electrons is dependent upon the
charge of the nucleus.
Neutrons
(brahma
/
balance) :
Since protons are positively charged, there is an incredibly strong
repulsive force between all the protons in a nucleus. To counteract
this strong repulsive force, neutrons apply an even stronger force
on the protons in a nucleus (balance).
Electron
(shakti
/ mother nature)
:
Electron
is the only fundamental particle that takes active part in any chemical
reaction and so, results in the molecule or a compound formation.
This is the electron that is responsible for chemical reactions
and so gives birth to three different states of matter viz. Solid,
Liquid and Gases.
Number
of Electrons and their configuration (Commonly known as Electronic
Configuration) differs in various atoms and gives birth to so called
"Elements" (life).
Nucleus (vishnu), Proton (shiv), Neutrons (brahma) and
Electron
(shakti) :
The chemical reactivity of an atom is dependent upon the number
of electrons and protons, and independent of the number of neutrons.
Yantra
with Physics :
I
am trying my best to make this as simple as possible so that everyone
can understand.
A God and Goddess is represented by a Mantra and a mantra is represented
by a Yantra. In order to understand the Yantra first we have to
understand the atom which involves Chemistry and Physics.
Atom :
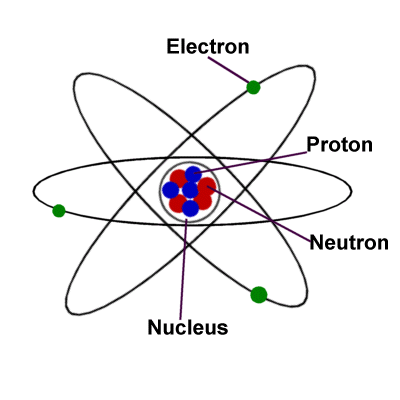
Atom
Atoms are the basic building blocks of ordinary matter. Atoms can
join together to form molecules, which in turn form most of the
objects around you.
Atoms are composed of particles called protons, electrons and neutrons.
Protons carry a positive electrical charge, electrons carry a negative
electrical charge and neutrons carry no electrical charge at all.
The protons and neutrons cluster together in the central part of
the atom, called the nucleus, and the electrons 'orbit' the nucleus.
A particular atom will have the same number of protons and electrons
and most atoms have at least as many neutrons as protons.
Atomic Number :
The atomic number uniquely identifies a chemical element. In an
atom of neutral charge, the atomic number is also equal to the number
of electrons.
Elements
:
Elements consist of only one kind of atom and cannot be decomposed
into simpler substances.
All versions of the periodic table include only chemical elements,
not mixtures, compounds, or subatomic particles. Each chemical element
has a unique atomic number representing the number of protons in
its nucleus. Most elements have differing numbers of neutrons among
different atoms, with these variants being referred to as isotopes.
For example, carbon has three naturally occurring isotopes: all
of its atoms have six protons and most have six neutrons as well,
but about one per cent have seven neutrons, and a very small fraction
have eight neutrons. Isotopes are never separated in the periodic
table; they are always grouped together under a single element.
Elements with no stable isotopes (Isotopes - One of two or more
atoms having the same atomic number but different mass numbers)
have the atomic masses of their most stable isotopes, where such
masses are shown, listed in parentheses.
In the standard periodic table, the elements are listed in order
of increasing atomic number (the number of protons in the nucleus
of an atom). A new row (period) is started when a new electron shell
has its first electron. Columns (groups) are determined by the electron
configuration of the atom; elements with the same number of electrons
in a particular subshell fall into the same columns (e.g. oxygen
and selenium are in the same column because they both have four
electrons in the outermost p-subshell). Elements with similar chemical
properties generally fall into the same group in the periodic table,
although in the f-block, and to some respect in the d-block, the
elements in the same period tend to have similar properties, as
well. Thus, it is relatively easy to predict the chemical properties
of an element if one knows the properties of the elements around
it.
As of 2013, the periodic table has 114 confirmed elements, comprising
elements 1 (hydrogen) to 112 (copernicium), 114 (flerovium) and
116 (livermorium). Elements 113, 115, 117 and 118 have reportedly
been synthesised in laboratories however none of these claims have
been officially confirmed by the International Union of Pure and
Applied Chemistry (IUPAC). As such these elements are currently
known only by their systematic element names, based on their atomic
numbers.
A total of 98 elements occur naturally; the remaining 16 elements,
from einsteinium to copernicium, and flerovium and livermorium,
occur only when synthesised in laboratories. Of the 98 elements
that occur naturally, 84 are primordial. The other 14 elements occur
only in decay chains of primordial elements.No element heavier than
einsteinium (element 99) has ever been observed in macroscopic quantities
in its pure form.
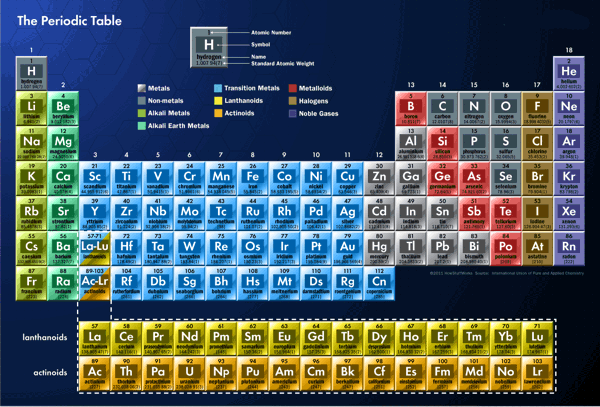
Periodic
Table (click on the image to enlarge)
Decoding
the triangles in Yantra :
There are total 43 Triangles in a Shri Yantra. In periodic table
Element 43 is technetium
Technetium :
Technetium is the chemical element with atomic number 43 and the
symbol Tc. It is the lowest atomic number element without any stable
isotopes; every form of it is radioactive (Material that emits radiation
energy in the form of alpha, beta, or gamma particles or rays).
Nearly all technetium is produced synthetically, and only minute
amounts are found in nature. Naturally occurring technetium occurs
as a spontaneous fission product in uranium ore or by neutron capture
in molybdenum ores. The chemical properties of this silvery gray,
crystalline transition metal are intermediate between rhenium and
manganese.
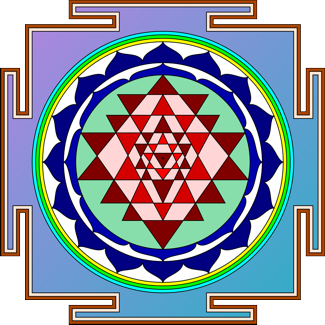
Shri
Yantra
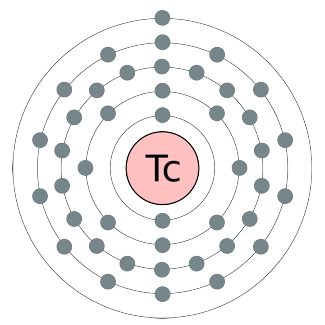
Technetium
The Image of Shri Yantra and of Technetium is similar.
By this we can come to a conclusion that Yantra is a symbol representing
a specific chemical element of Atom.
The
petals around the triangles is explained as below :
Bounce
Motion :
Some particles do move along the field lines of the Earth. The crowded
magnetic field lines near the poles cause particles to "reflect"
and move back from the same direction from which they came. They
bounce back and forth from one pole of the Earth to the other.
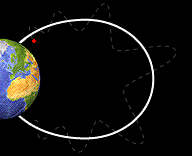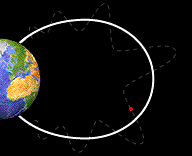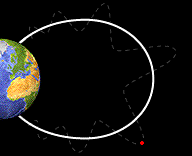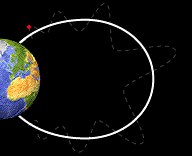
Particle
bouncing back and forth from one pole of the Earth to the other











