| OPTICAL PROPERITES OF GEMS Optical properties are those which are related to the behavior of light, on, or in, a gemstone.
Some of these can be seen, and even quantified, with the naked eye alone. Three such characteristics are: luster, transparency, and color. The study of these factors, and their use in gem identification and evaluation, is sometimes called optical gemology.
Other characteristics are revealed, or measured, only through the use of special instruments. Some of these include: refractive index, optical character, birefringence, pleochroism, dispersion, reaction to ultraviolet light and selective absorption. When these properties of gems are analyzed and measured, one is engaging in laboratory gemology.
Luster :
The luster of a gemstone is comprised of the quantity and quality of the light reflected from its surface. There is an inherent, potential luster possible for each species and variety of gemstone. The actual luster, on any individual piece, however; may be less than this, due to the skill level of the lapidary or facetor, the presence of inclusions, or various chemical or physical changes, such as oxidation or abrasion, that can affect the surface.
The names, which have been given to the various lusters seen in gems, are derived from their resemblance to familiar surfaces. (The prefix sub- indicates "just less than".) Some lusters are so embodied by a particular stone, that its appearance is named for that stone, as in the case of adamantine luster (adamas = Greek for diamond), and pearly luster. Looking through either of your textbooks at the descriptions of the various gems will convince you that a substantial majority of gems have a glass-like or "vitreous" luster.
Look at the picture of the fire agates below and compare what you see on their surfaces to that which you'd see on a freshly washed and dried drinking glass keeping that image in mind should help greatly in estimating the luster of a gem.
Pyrite (in shist) : Metallic
Diamond : Adamantine (like diamond)
Zircon : Subadamantine
Fire Agate: Vitreous (like glass)
Fluorite : Subvitreous
Nephrite Jade : Greasy
Amber : Resinous
Pearl : Pearly
Tiger's Eye : Silky
Granite : Dull Transparency :
Technically known as "diaphaneity", the degree of transparency of a gemstone is one of its most directly observable and familiar characteristics.
Transparency (or lack of it) is dependent on how much light gets through the gem, and is affected not only by the chemical and crystalline nature of the gem, but also by its thickness and, as in the case of luster, by inclusions, and its surface condition. In the discussion and examples that follow below, we will be looking at the "potential" maximum transparency of a species in general, rather than the actual transparency of any individual specimen.
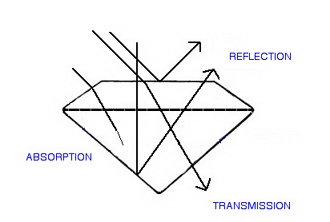
When light hits the surface of a gem, there are only three fates for it (with respect to transparency). Various portions of the total amount of light will be reflected, absorbed or transmitted. The proportion in each category will determine the transparency of that gem.
Three fates for light hitting a gem : It can reflect (be returned) from the surface or interior of the gem, it can be absorbed by the gem, or it can be transmitted through the gem Reflection :
Light is reflected when it hits an exterior or interior surface of the gem and is bounced back off, or out of, the gem, in the direction of the observer.
Absorption :
When light enters a gem and does not exit, we say it has been absorbed. Light is a form of energy, and energy does not just disappear, instead the visible light has been converted to a non-visible form of energy, in most cases, heat.
Transmission :
Light that travels through the gem and exits in a direction other than that of the observer, is said to have been transmitted.
The issue of transparency (with the factors of reflection, absorption and transmission) is actually more complex than it may seem at first, because it is intimately linked with the color characteristics of a gem. For the time being, however; we can be satisfied with the following descriptions:
Opaque :
No light is transmitted.
Translucent :
Some light is transmitted.
Transparent :
A high proportion of the light is transmitted.
The term "semi" is sometimes added to describe intermediates, and gives additional categories beyond the basic three.
Citrine : Transparent
Prehnite : Semi-transparent
Chrysoprase : Translucent
Sugilite : Opaque Within
any particular species of gem, it is often the most transparent
pieces which are the most valuable. For example, in chrysoprase,
shown above, which is generally semi- to fully translucent, one
finds occasional pieces that are semi-transparent. These are greatly
admired and sell for higher prices. The same can be said of nephrite
and jadeite jades where price (within the same color) can escalate
dramatically based on nuances of transparency. Likewise, in gems
that are usually opaque, like the sugilite pictured above, the
occasional semi-translucent to translucent piece (called "gel
sugilite"), is highly prized.
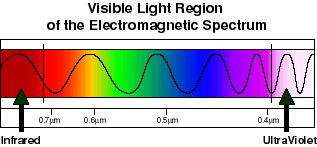
Scientists in later years, were able to show that the color of light is a function of its wavelength. In the diagram above, a wave-form representation shows the relative distance from crest to crest (wavelength) of the various components of white light. Notice that these distances increase toward the red end of the spectrum and decrease toward the violet end. The wavelengths are very small, and we do not have everyday measurements to describe them. A nanometer (nm) is one billionth of a meter, and is an appropriately sized unit for this use. Using this terminology, then, the portion of the electromagnetic energy spectrum which our eye and brain interpret as light, extends from approximately 700 nm on the long (red) end to about 400 nm on the short (violet) end.
(For generations, students have been introduced to "Mr. Roy G. Biv", as a simple device for remembering the order of the colors in the light spectrum). Not to get too far afield from our subject matter, it is necessary to mention that this spectrum extends greatly on either side of the narrow visible range: into ultraviolet, xrays and gamma rays on the short end, and into infrared, microwaves and radiowaves on the long end. The little segment of it that we are concerned with in this class, not only powers vision, but also photosynthesis, and many other biologically relevant processes. It is also important to point out that the energy content of the various colors of light is related, in an inverse way, to their wavelength. That is, light of shorter wavelength is more energetic than light of longer wavelength.
Selective Absorption: The color of most objects, gems included, is a result of a process called "selective absorption". Let's take an example: suppose you have on a yellow shirt, Why is it yellow? The fibers and dyes in it absorb only some of the wavelengths of the white light that hits them, primarily in the red, orange, green, blue and violet bands. The wavelengths that are left (the yellow ones) are reflected back to the eye of the observer whose brain interprets light energy of that wavelength, as what we call yellow. I'm sure you can see how a shirt could be greenish yellow or orangey yellow if wavelengths slightly shorter or longer than yellow were also reflected, and red or blue if it had a quite different pattern of selective absorption. With opaque objects it is the color of reflected light that we see, with transparent and translucent ones, the color we see consists of a mix of both their reflected and transmitted wavelengths.
Let's see if we can put together the information on transparency with that on color :
Ok, so selective absorption determines color, but what, then, determines selective absorption, you ask? That is, why, precisely, do rubies look red and sapphires look blue? The basic answer is simple, and two-fold, and goes right back to the basic point previously made in Lesson 3 regarding all gem properties. Selective absorption in gems is determined by an interplay between their chemical makeup, and their three dimensional structure.
The atoms (or ions) which create color in a gem are called "chromophores". Some of the most common chromophores in gemstones are: atoms of titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, nitrogen, and boron and their various ions.
(A new, undefined term, "ion", has crept in here, so let's deal with that). Atoms are made of smaller particles: protons, neutrons and electrons. The protons have a positive charge (+) and the electrons a negative one (-). In an atom, such as oxygen, or iron, or any other, the number of protons and electrons are equal making it, overall, a neutral body.
Suffice it to say, that chemical and physical events can, and do, occur that add or subtract electrons from atoms, making them into negatively or positively charged bodies called ions. Fe is the chemical symbol for a neutral iron atom, Fe+2 designates an iron ion (an atom of iron which has lost two of its electrons), Fe+3 has lost three, Cl- is a chlorine atom that has gained an electron, etc. The main point for you to understand is that events that occur in the "life" of gems and minerals (or in a gem enhancer's laboratory) can change the ionic state of their constituent atoms and ions, and thereby affect their color.
Back to the main point, the presence of various chromophores, as well as certain details of the three dimensional structure of the material itself, cause the selective absorption, which, in turn, causes color. To put it another way, both the presence of particular atoms and ions, as well as specific crytal "defects" such as missing atoms or extra ones, areas of compression or strain, can act as the agents of color in gems.
Idiochromatic vs Allochromatic Gems :
With regard to the source of their color, gems fall into two categories: idiochromatic and allochromatic. Idiochromatic gems derive their color simply from the chemistry of their basic formula. Due to this fact, such gems will always occur in various shades of the same basic color. The other group (more common) are allochromatic, meaning that the chemistry of their basic formula does not cause any selective absorption so in the pure state, they are white or colorless. In gems of this sort it is tiny, trace amounts of impurities that act as the chromophores. Such gems occur in colorless forms as well as in a variety of other colors depending on the nature and amount of the "contaminants" in them.
[I think you'd probably get an argument from someone who is admiring their beautiful blue sapphire, if you called the tiny amounts of titanium and iron that give it that color,"contaminants", though.]
Some examples of idiochromatic gems are: peridot containing iron, (Fe), rhodochrosite with manganese (Mn) and cuprite and malachite containing copper (Cu).
Idiochromatic Gems :
Rhodocrosite (Mn)
Cuprite (Cu+1)
Malachite (Cu+2) Hey wait a minute, you say cuprite is red, malachite is green, and both contain copper! What gives? Welcome to the wonderful world of gem color! It is not quite as simple as: this element makes this color, and that element makes another color. Each gem's color is determined by an interplay between its chemical makeup (including the ionic state of its chromophores) and its structure.
To further pursue this point: some emeralds are green due to chromium content, while some get their green color from vanadium. So, iron (as in peridot), copper, chromium or vanadium can each be responsible for "greenness" in a gem. But on the other hand, chromium in corundum makes red rubies, and iron in chalcedony, makes orangey carnelian, but in sapphires gives us yellow. Futhermore, green zircons and green diamonds get their color not from chromophores, but from crystal defects.
Allochromatic Gems :
Some examples of allochromatic gems are: beryl, corundum, quartz, grossular garnet, tourmaline, topaz, spinel and nephrite jade. In some cases the "pure" material is the most common and therefore the lowest in value (corundum, quartz, beryl and topaz are in this category); but in others, the pure form is so rare as to be a high value collector's item. This is especially true in the case of grossular garnet, tourmaline and nephrite jade. Colorless spinel is so rare that it literally has not been found in Nature; we know it can exist, though, because colorless synthetic spinel is made in labs.
A good example of an allochromatic gem species is corundum. Pure Al2O3 is colorless, as in white sapphire, but if we add just a tiny bit of iron to the mix then we get yellow or orange fancy sapphire, pair the iron with a bit of titanium, and the gem is the familiar blue, and if chromium is the chromophore, then the corundum is red and called ruby.
Allochromatic Gems (in their pure state) :
Colorless Beryl (Goshenite)
"White" Sapphire
Colorless Quartz (rock crystal)
Colorless Grossular Garnet (leucogarnet) Allochromatic Gems (in their impure state) :
Beryl : Emerald (chromium or vanadium)
Corundum : Sapphire (titanium and iron)
Quartz : Carnelian (iron)
Garnet : Spessartite (manganese) Other Sources of Color :
Some gems get their color (or apparent color) from visible to microscopic inclusions of other minerals within them. One of the most beautiful of all the chalcedonies, often called "gem silica" but more properly termed "chrysocolla chalcedony" has a vivid blue-green color. The minute quartz crystals are actually colorless, but in amongst them are tiny crystals of the blue green (very soft) mineral, chrysocolla. The overall impression, in the best specimens, is a translucent chrysocolla colored gem, with the durabililty of quartz.
In the varieties known variously as strawberry and raspberry quartzes, visible particles of red or red-orange hematite in the colorless quartz, create a pink, orangey, red or polkadot looking gem, depending on their size and number.
Inclusions :
Chrysocolla Quartz
Strawberry Quartz Patterns in color : banding/zoning :
One of the most common features of some of the aggregate gems is the presence of patterning. Since these gems are formed from very tiny single crystals, we can easily envision conditions where differently colored pools or batches of tiny crystals mix and intermesh creating bands, dots or other patterns. Agates and jaspers are the most commonly seen gems with strong patterns.
It frequently happens that single crystal gems subjected to changing conditions during their growth can also show bands or zones of different colors or shades of the same color. When these are dramatic and attractive, they are desirable, but far more commonly, gems of this type have nondescript, patchy, or zoned coloration, and are considered inferior to more evenly colored pieces.
Aggregates With Patterns :
Zebra Agate
Picture Jasper
Mookaite Jasper
Carnelian
lavendar Agate
Dalmation Jasper
Rain Forest Jasper Single Crystal Gems with Attractive Color Zoning :
Ametrine
Multi-color Tourmaline
Watermelon Tourmaline Color Descriptions in Colored Gemstones :
There are three aspects to a formal colored stone color description: hue, tone, and saturation. Using these three descriptors, very detailed and nuanced color discriminations can be made, and communicated, between gemologists, jewelers and gem buyers. Let's take them each in turn :
Hue :
The hue of a gem is its basic position in the color spectrum: red, orange, yellow, green, blue or violet but it also includes all the possible intermediates like slightly yellowish orange, or moderately bluish green.
Tone :
The tone of a gem, basically how light or dark the color, is independent of its hue and ranges from so light as to appear virtually colorless, to so dark as to look black.
Saturation :
The least commonly quantified aspect of gem color is "saturation", which is a measure of the purity of color, that is, the relative presence or absense of modifying grey or brown hues. It turns out that in most cases, as long as the hue and tone are reasonably nice, it is the degree of saturation of color that is the prime value setter in gemstones.
You might think as to why does color description need to be so formalized? The main reasons are listed below :
One well done, and widely used, system for color description is that developed and taught by GIA (Gemological Institute of America). Although not universal, it is familiar world-wide, and the basis for most formal gem description and evaluation in the US and Europe.
Since the wavelengths and light colors grade into one another in infinitesimal changes, there are an essentially infinite number of hues which could potentially be described. Most of these hues would be indistinguishable from each other to our eyes, so GIA has settled on a group of 31 which humans with normal color vision (and some training) can discriminate. The set of plastic gem models below is a representation of those 31. (It should be noted that GIA has taken some liberties with the traditional "Roy G. Biv" spectral colors, deleting indigo, and adding purple after violet. In the set below, then, you see: red, orange, yellow, green, blue, violet and purple. The intermediates are described by terms like slightly, moderately and strongly to indicate a spectral hue modified to various degrees by those on either side of it on the spectrum. It also recognizes hues which are exactly 50/50 mixes such as red-orange and blue-green.
HUE :
Gia's 31 basic gem hues The hue, then, consists of the key (or spectral) color plus adjectives describing the hue and strength of the secondary modifying color, if any. For example: slightly purplish red, symbolized by "sl p R" (sl for degree, lowercase p for the secondary hue (purple), and upper case R for the primary hue, (red). The description strongly yellowish green: "st y G" would be decoded using the same logic. Once you have been trained to see nuances of color, you recognize that pure spectral colors in gems are quite rare, and as a result, costly. For example: a hue of simply "B" would be pure spectral blue and if other factors of color and clarity were good, the piece would command a premium price.
If you are thinking that it looks like there are more gradations in the blues and greens in the display above, than in the other colors, you are right. Human vision has finer powers of color discrimination in that part of the spectrum, which this system takes into account.
TONE :
Each of the 31 hues exists in a range of tones from almost colorless to almost black. GIA labels the tones as 0 - 10. {0 ( appears colorless), 1 (extremely light,) 2 (very light), 3 (light), 4 (medium light), 5 (medium), 6 (medium dark), 7 (dark), 8 (very dark), 9 (extremely dark), 10 (appears black).
The figure below represents the 2-8 part of that range, which is, in the great majority of cases, the range for marketable colored gems. For most species the most valuable tones are in the 5-6 range. The set below is shown without hue, and it takes practice and patience for the would-be colored gem grader to learn to superimpose hue onto these, and get a valid tone reading.
An additional complication comes from the fact that gem species differ in their inherent tone ranges. For example, let's compare an aquamarine and a pyrope garnet each of tone 6. Objectively, each is exactly the same, but that depth of color is about the deepest that will ever be found for aquamarine and the about lightest possible for any pyrope. One should not be surprised, then, to find the aqua dealer calling her stone "very dark" and the garnet seller raving about how beautifully light his stone is when they are both "6"'s.
GIA's tone scale from 2 (very light) to 8 (very dark) SATURATION :
Finally, it's time to examine the most subtle aspect of gem color, saturation: in a manner of speaking, this measure is the degree to which the other spectral colors "muddy up" the main hue. Think of a can of pure red paint and start adding in various amounts of all the other colors the more you add of the other spectral hues, the "browner" the red will get. Now do the same thing with a can of pure blue: the more you add the "greyer" the blue will get. In general, desaturating "warm colors" makes them look brownish while the same effect in "cool" colors looks more grey. Therefore, GIA's system of describing saturation makes a distinction between cool and warm hues.
Warm hues = green through red (desaturated to brown) Cool hues = purple through blue (desaturated to grey)
Six degrees are recognized ranging from : 1 (brownish/greyish), 2 (slightly brownish/greyish), 3 (very slightly brownish/greyish), 4 (moderately strong), 5 (strong), 6 (vivid)
In the figures below, you can get a better idea of the saturation effect by looking at the flat end of the plastic gem replica, rather than the "gem part".
GIA's six degrees of warm hue saturation
GIA's six degrees of cool hue saturation When giving a gem's formal color description in words, then, the gem below might be said to be: medium dark, slightly greyish, blue-violet. It sounds more natural to put the tone, saturation and hue in that order. In a numeric description as required in offical gem grading documents, however: the order would be: hue, tone and saturation, thus: BV 6/2.
Iolite : In words : Medium dark, slightly greyish, blue-violet = official "grade" : BV 6/2 One final point on the GIA color grading scheme: Two non-spectral colors are used (in addition to the officially sanctioned 31) and those are pink (pk) and brown (br). If one were to strictly follow the GIA system, all shades of pink are really lighter tones of red, and brown is simply desaturated orange. It is rather a matter of bowing to tradition and convenience to recognize pink and brown as "colors" in their own right. You will see evidence of this practice in the color description below.
Spinel : Medium Dark, Moderately Orangey, Strong Pink Mod O PK 6/5 Color Grading in Diamonds :
You will recall from Lesson 1 that within the gem industry, there are separate systems for marketing, grading, and describing colored gemstones and diamonds. For virtually all natural diamonds, discernable color is a negative attribute. The closer it is to an absolutely colorless condition, the more highly valued is the gem.
In the case of what are called "fancy" diamonds, whose color is both intense enough, and attractive enough, to be desirable, color is described and evaluated in a similar manner to that used for colored stones. There is sort of a "U" shaped value curve for diamonds, whereby the highest values accrue to only the whitest, and then, again, to the most vividly colored specimens, with value bottoming out in the central ranges where there is just a bit, to a moderate amount, of color.
Although most of the diamonds you might see on a day-to-day basis are called "white" and appear so, a little study and comparison will verify that a truly colorless diamond is a thing of great rarity, and the vast majority of diamond gems are actually tinted with small but noticeable amounts of yellow or brown.
I was taking some liberties, perhaps, by using the GIA system in the preceding discussion of colored gem descriptions, but without doubt, the GIA system is the one to learn if you are interested in diamond colors and value. It is understood everywhere in the world, and used for formal grading in most countries. Even competing gem grading laboratories either use the GIA system, or provide a key to translate theirs to it. For example AGS, The American Gem Society uses 0 - 10 for their color grading scale (0 = D, 0.5 = E, 9.5 = W, 10 = X - Z, etc.), but gives the customer an exact conversion scale to the GIA system with their reports.
Before the GIA system was developed (beginning in the 1930's), there were as many diamond color descriptions as there were diamond sellers. Many of them used A, B, C and A+, AA, AAA etc. while others used adjectives like "river" and "cape". It is easy to see how difficult it would be to have a reliable system for trade under those conditions. GIA's scale did away with A, B and C because of their long histories and diverse useages, and developed a system based on color grades from D-Z for "colorless" stones, plus the term "fancy" to indicate those whose strong color made them more, rather than less, valuable.
What the Letters Mean :
D, E, F :
Gems in this range appear colorless even in larger sizes, only a highly trained diamond grader can tell the differences between them.
G, H, I :
These grades describe gems that look colorless to most viewers in smaller sizes and if mounted.
J, K, L :
Small and mounted stones of these grades look near colorless, but larger and unset gems begin to have noticeable color
M-Z :
Gems in this range are worth much less than higher color grades and range from some color noticeable to distinctly light yellow (or brown).
Z+ :
Beyond Z is the range of the "fancy" diamonds whose value is based on their hue tone and saturation, as in colored stones. In general browns are least valuable with yellow, orange, and green worth considerably more. The pinnacle of value for naturally colored diamonds is occupied by purple, blue, pink, and at the very tip-top, red.
The images below may or may not be enlightening, based on the characteristics of your vision, viewing circumstances, and monitor calibration, but they will hopefully serve to illustrate at least some of the aspects of our topic.
How Do they Do That ?
After straining your eyes to see the minute changes in the illustrations above, you might ask, how can a grader do it, especially when a great deal of money rides on the difference between, say an F and a G grade, or an L and an M? I might joke that the answer is "verrrry, carefully". In actuality, not everyone can become a successful diamond grader there is both exacting training and "raw talent" involved. In practical terms, the mechanics of the process is that the stones to be color graded are serially compared to the gems in a special "master stone" set, under controlled conditions of lighting and viewing.
Gemology Then and Now :
Pre-modern :
What we could refer to as modern-style gemology began and developed in the period between 1930 to 1950. Prior to that time gemology was a simpler discipline to master and practice than it is today. The mains reasons for this are :
To give an example, I'll relate a true story: Recently, a client came to a gemologist I know, with an antique ring that they had inherited. Along with the ring was a jeweler's certificate, dated 1870, certifying that the gem was an emerald. A variety of tests soon revealed that the stone was, in fact, a green tourmaline. Had the client's ancestor been duped by an unscrupulous jeweler? Most likely not. With the tools and knowledge available in the 1870's, a green stone of hardness greater than 7, and with a vitreous luster that came from South America (as this one did) would have been called an emerald. Thus it is, that a lot of the "rubies" in museum collections are actually red spinels, and much of the "jade" pieces among the displayed artifacts are serpentine or hydrogrossular garnet.
Gemology Today :
Today's gemology is a very different sort of game I say "game" because it has quite literally become a tug of war, or an "arms race", if you will, between gemologists and those who seek to profit from misinformation or ignorance. In comparison with the list above we find:
Thus, today's
gemologists, as much as those in any other rapidly advancing area
of science, must constantly watch the literature, and keep themselves
abreast of any new tools or techniques which they can use to keep
"one step ahead" of those who would deceive. As we turn our attention
to laboratory gemology, we'll be looking at several basic properties
or "behaviors" of light and seeing how each, in turn, is useful
in describing gemstones. The aim of this part of the lesson is
to acquaint you with first, the basic facts of these properties,
and subsequently show how they are measured, and/or how they can
be used to solve problems in gem identity.
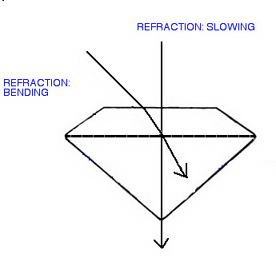
We all know that the "speed of light" is the stuff of which cosmic measurements are made, and you may or may not have that famous figure in your store of readily retrieved facts. When we discuss the speed of light in an astronomical context we are thinking of the speed of light in a vacuum (the absense of matter), but light slows down when it travels through any medium denser than a vacuum.
Air is not a vacuum, so light slows as it enters our atmosphere from space, in the same way, light slows from its "air speed" when it enters any material of greater density than air, which would include all gemstones. If a light ray enters the gem at any angle, other than directly perpendicular, to a surface, then it also bends as it slows. The degree of slowing is determined by the density of gem, and the degree of bending is determined both by density and by the angle of its entry.
The ratio of the speed of light in air, to the speed of light in a gem, is called the gem's refractive index or RI. It differs, sometimes dramatically, between gem species, and is one of the most useful gem identification criteria. In natural gems it ranges from about 1.2 to 2.6. and can, in most cases, be measured by an instrument called a refractometer.
Refraction includes the slowing and the (usually) consequent bending of light as it enters a gem Behavior of Light 2 : Dispersion
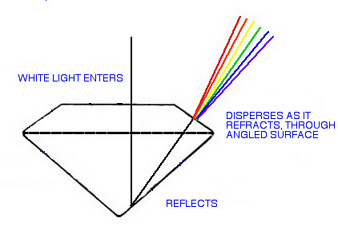
Dispersion (sometimes called "fire"), is the separation of white light into its spectral colors. It may be observed as specks of red, blue or green which flicker as the gem is turned.
The cause of this phenomenon is the differential refraction (bending) of the various wavelengths of light as they travel through a gem. Red (long wavelength) bends less, violet (shorter wavelength) bends more. This causes the colors to become separated. Although dispersion, theoretically, occurs in all gems, the degree depends on the RI of the gem material, and only those gems with sufficiently high RIs, have dispersion which is pronounced enough to be actually visible.
The exact figures for dispersion can be painstakingly measured in a laboratory setting using special equipment, and then calculated as the difference between the RIs of red light and violet light in a given species. Potential dispersion in gems, thusly measured, ranges from .007 to .280.
Outside the lab, dispersion is generally judged visually, without instruments, simply as: absent, slight, moderate, strong or very strong. The degree of visible dispersion is affected by species (due to RI), but also by the body color, and cut proportions of the gem. In general, the denser the gem, the greater its potential dispersion. Light body color, and steep crown angles enhance the display, whereas dark body color and shallow crown angles diminish it.
Dispersion of white light as it leaves a gem Examples of gems with slight dispersion potential are : fluorite (.007), common glass ("crown" or silica glass) (.010), and quartz (.013). Regardless of color or cut, these gems just aren't going to show visible dispersion, the effect is too slight.
Those with moderate potential for dispersion include: tourmaline (.017), corundum (.018) and spinel (.020). Such gems rarely show visible dispersion, but an occasional light colored specimen of substantial size with very high crown angles may do so.
Examples of strongly dispersive gems are: zircon (.038), diamond and Benitoite ( both .044), and cubic zirconia (a synthetic), (.066). Gems in this range will usually show dispersion. Exceptions might be those of very dark body color, or small pieces cut with rather low crown angles.
Very strongly dispersive gems include: sphalerite (.156), strontium titanite (a synthetic) (.190) and synthetic rutile (.280). There would be very few cases where a gem in this group did not show substantial dispersion.
Benitoite and sphalerite both with cuts and body colors which permit their substantial dispersion to show
Diamond a gem admired for its dispersion Diamond is the most well known gem that shows dispersion, and it is one of that gem's most appealing attributes. The success of either a natural or man-made diamond simulant, depends to a large extent on how well the substitute matches diamond in this characteristic.
Before the post-1950's crop of synthetic diamond simulants came on the market, the choices were limited to glass, foil-backed glass, or amongst crystalline gems: white sapphire, white beryl, white topaz or white zircon. Of that group, zircon made the best simulant due to its dispersion being much closer to that of diamond than any of the others.
Quite a few synthetics have been created since then, but, only one, like the Baby Bear's porridge, is "just right" and that is cubic zirconia. Even though its dispersion figures are a bit too high, in the small sizes usually encountered, the difference is not obvious. YAG on the other hand is without noticeable dispersion and looks very "glassy", while synthetic rutile and strontium titanite have way too much to look convincing.
The first set of pictures below shows, respectively, the two most convincing natural and man-made simulants. The second set shows a group of three temporarily popular, but unconvincing synthetics.
White Zircon (natural)
Synthetic Cubic Zirconia
YAG
Sythetic Rutile (less)
Sythetic Strontium Titanite (more) Behavior of Light 3 : Light is Influenced by the Optic Character of a Gem
There are two groups of gems with regard to light refraction, each is said to have a different "optic character": SR or DR.
SR
stands for singly refractive. In such gems, each beam of light
entering the gem stays as a single beam which has a single refractive
index (travels at the same speed), regardless of the direction
from which it enters. In this group we find all amorphous gem
materials, such as opal, glass, amber, etc. as well as all crystalline
gems belonging to the cubic (isometric) system. The most commonly
encountered gems of the cubic system are: diamond, garnet and
spinel.
Garnet
Spinel (cubic system)
Opal (amorphous)
DR stands for doubly refractive. In such gems, single beams of light upon entering the gem, are split into two separate beams, which then travel perpendicularly to each other. Each of the resultant beams takes a different path through the crystal and, consequently, has its own speed. Such gems, then, have two RIs, one for each half of the original beam. In this group are all the gems of the non-cubic crystal systems. DR Gems :
Amblygonite (triclinic)
Citrine Quartz (trigonal)
Pearl (orthorhombic)
Scheelite (tetragonal) Important Note :
Each DR gem, based on details of its crystal structure, has either one or two directions in which the light entering it behaves as if the gem is SR. These directions are known as "optic axes". Those species with a single optic axis are known as "uniaxial" and those with two are, logically, called "biaxial". It is sufficient to simply note this at present, but we will return to this fact and see that the existence of optic axes can constrain the methods by which we test some of the optical properties of gems, as well as serve as a valuable identification criterion in its own right.
Birefringence :
BR :
Birefringence, a property of DR gems only, is measured as the difference between the high and low RIs of the split beams. It ranges from a low of .003 to a high of .287.
When a transparent gem with high BR is faceted, and the view through the table direction of that gem is not in an optic axis direction, the slightly "out of sync" light beams can create an appearance of interior "fuzziness" or in larger stones, can show up as two distinct images of each facet edge. This is known as "facet doubling" and it can be a pain in the neck to a facetor who, in trying to prevent it, must find an optic axis direction for the table of the stone. But it can also be a valuable identifying characteristic that can often be seen with the naked eye or a simple 10x loupe.
For reasons unknown to me (perhaps it is a "British-ism"), the Hall text abbreviates birefrigence as "DR" in the tables at the back of the book and on the individual species pages. BR as used in this lecture, is the standard abbreviation used in most books.
Calcite
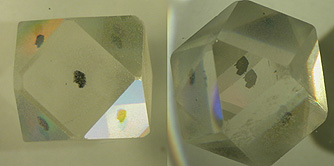
Gems with high BR, showing facet doubling The pictures below demonstrate the effect very clearly. The material below has been cut into a geometric form known as a "cuboctahedron" from synthetic rutile (which has extreme birefringence = .287). It has been placed above a single black ink dot on the surface. The left photo shows the view through the central face which has been cut on an optic axis. Note that the dot is clearly visible as "one" as are the reflections of its image in the other faces. In the second photo the piece of rutile has been turned so that we view the dot through another of its faces (one not on an optic axis). Now we see two images of the dot.
Synthetic rutile above a single black dot : viewed in the optic axis direction, viewed in a non-optic axis direction Note :
The newest diamond simulant on the market is called Moissanite. (That's the one that required the new generation of electrical conductivity testers). Fact : Moissanite is markedly birefringent; diamond, being SR, has no birefrigence.
Behavior of Light 4 : Pleochroism
As we know, DR gems split light into two perpendicular rays, each taking different paths through the crystal : one ramification of this is birefringence, another is pleochroism. Pleochroism is the property of DR gems which results in their showing different colors, or different shades of the same color, when viewed in different crystal axis directions.
How can this be? Think again of the crystal lattice of a DR gem, made of carefully laid out atoms of the gem's component elements (and the trace chromophore elements), with fixed distances and densities that can vary depending on direction. If two beams of light take a different path through this lattice, they may then be affected differently by selective absorption and emerge with different colors.
This effect can be weak, moderate or strong, depending on the species of gem, the colors involved, and also on the color tone of the particular piece. A very light piece of a pleochroic species will show the effect less clearly than a more richly colored one. Unless the effect is extreme (as it is in iolite and Andalusite), we generally do not see it in a cut gem, because the bouncing and mixing of the light caused by the internal reflections from facets and edges blends the colors together and obscures it.
Dichroic gems (like corundum) show two different colors while trichroic gems (like iolite) show three.
Pleochroism will not be observed in SR gems, nor in DR gems when looking through an optic axis direction.
In most cases, pleochroism can best be observed using an instrument called a dichroscope. This cleverly made little tool uses a piece of highly birefringent colorless calcite to split the incoming light into two beams which are bounced off tiny mirrors positioned inside so as to reflect each of the two beams onto a pair of side-by-side viewing windows. This placement allows the viewer to simultaneously see light that has traveled two different paths through the gem or crystal being viewed. Simultaneous viewing isn't absolutely necessary, but considering that these effects are often subtle and color memory is poor, it is certainly easier that way. All that is necessary to use this instrument is a good light source, and a transparent to translucent gem.
Below you see a dichroscope (about 2" long) and a simulated view of a ruby gemstone, as it would appear through the dichroscope. For the sake of clarity I have emphasized the color differences, but they are not quite so distinct in reality. Ruby has an orangey red color axis, and a purplish red color axis.
Calcite Dichroscope
Simulated view of ruby's characteristic pleochroism as it would appear looking through a dichroscope One must view the gem being tested with the dichroscope from several different directions because :
1. Some directions will be optic axis directions, in which there would be no pleochroism shown, even if the gem were pleochroic. So, if you based your conclusion on one direction only, there would be a large chance for error.
2. Only two colors show at a time, so, although dichroism might be detected from a single directional view, it would take more than one to see all three colors in a trichroic stone.
When strong, pleochroism may complicate the orientation process for a cutter and/or create setting issues for a jeweler. The cutter is going to want the most desirable or attractive color to be that seen when looking through the gem's table. For example, iolite has a lovely blue-violet axis, one that is grey, and a third that is a near colorless light yellow. Few buyers are interested in a grey or nearly colorless iolite.
A large number of tourmaline stones have one axis that is an opaque black! The other directions may show a lovely green or pink, but if the gem is not cut so as to prevent light bouncing from the black direction into the green or pink, the color in the finished stone will look terrible--> a muddy brown. To prevent this, a special "tourmaline cut" has been devised whereby the sides of the offending axis are cut so steep (approximately 70 degrees) that light from it is prevented from reflecting back into the gem. This leaves a gem with proportions that do not fit into standard prong or bezel mounts. Jewelers have had to devise a special "tourmaline mount" (as seen below) to accomodate such gems.
Iolite showing a lot of its undesirable grey axis
Iolite oriented to show a near ideal blue-violet
Tourmaline cut gem mounted in "tourmaline mount" Tanzanite gems, which in the rough are trichroic, but after their standard heating process become dichroic, have a blue and a purple axis. Blue stones have a higher per carat value than purple ones, but, unfortunately, the shape of this gem's crystals dictate that the greatest yield comes from cutting a purple gem. The cutter, then, must balance these two factors and try to orient the stone so as to give the largest, best colored, and most valuable stone from an individual piece of rough.
In some gems, most notably Andalusite, all the colors are attractive (brownish shades of green, red and yellow) and the mix of them in the finished gem is considered desirable.
Tanzanite with the purple color dominating
Tanzanite with the blue color dominating
Andalusite showing patches of all its colors The
tools you have are limited to a dichroscope and a gem reference
book that lists the pleochroic colors of gems. You find that
iolite is trichroic, sapphire is dichroic and spinel, which
is SR, shows no pleochroism. You find that ruby is dichroic
and, garnet being cubic, and glass being amorphous, are SR and
have no dichroism. You have no other equipment.
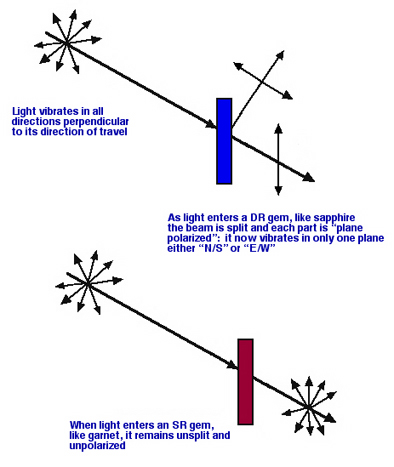
The rays of light from the environment or from standard man-made sources are vibrating in all directions perpendicular to their individual directions of propagation. They are said to be non-polarized. With respect to this vibration, light is affected by the gem materials it enters in two possible ways :
With DR materials, each of the two light rays that result from the splitting of an original beam, now vibrates in only one plane: it is said to be "plane polarized". Light goes into the gem non-polarized and comes out polarized. (The exception is light that travels through such a gem in an optic axis direction.) When we talk about such a pair of polarized rays, it is convenient to call one the E/W ray (East/West) and the other the N/S ray (North/South).
SR materials have no such effect, the light remains non-polarized as it travels through the gem. That is, light goes in and comes out of the gem non-polarized. We can also picture this by saying that light comes into the gem vibrating in all directions and exits the same way.
This property is one that is useful in gem identification and can be readily detected with an instrument called a polariscope.
The effect of optic character on the polarization of light Brief Review of SR -vs- DR Gems :
In singly refractive (SR) gems, light which enters remains as non-polarized beams, and travels through all crystal directions at the same speed. There is one refractive index (RI), no birefringence and no pleochroism. The SR gems can be amorphous or those of the cubic crystal system.
In doubly refractive (DR) gems, light which enters splits into two perpendicular polarized beams. Each beam travels at its own speed and has a separate RI which depends on direction. Such gems have birefringence and may show pleochroism. A DR gem can belong to any crystal system, other than cubic. All DR gems have either one (uniaxial) or two (biaxial) optic axis directions in which they will behave as SR.
Hint :
when
it comes to crystalline gems, if you commit diamond, garnet
and spinel to memory as SR, then virtually all other crystalline
gems you are likely to run into are DR. So, what is the optic
character of topaz? amethyst? enstatite? diopside? etc. etc.
Easy: Are they diamond, garnet or spinel No!, then they are
DR.
Polariscope The easiest way to test a gem for optic character is by using a polariscope. It is composed of two polarizing lenses with a light source below them. Each lens transmits only light that vibrates in single plane, either N/S or E/W.
When the light in the base is turned on, normal, unpolarized light is produced, which becomes polarized, let's say N/S, as it passes through the lower polarizer on the base of the unit. The upper lens can be rotated freely. If the upper lens is parallel to the lower one (also N/S), then the light travels through it, and we see a lighted field. The picture below is a photo taken looking into the upper lens as just described.
If the upper lens is rotated 90 degrees, the N/S polarized light passed by the lower lens is blocked by the, now, E/W position of the upper lens. The view through a polariscope in this "crossed filters" position is shown below :
Polariscope in the "crossed filters" position The
DR Reaction :
The
SR Reaction :
It is logical for you to expect that since a polariscope tells the optic character of a gem, it should show either a reaction of either SR or DR when a gem is tested. Both SR and DR readings are possible, as described above, but there is a also a third possibility. Certain gems, when tested as described above, neither blink dark and light, nor stay dark. They are light as intially viewed and stay light no matter how they are turned! What is going on? A gem with this reaction is an aggregate. (Remember, an aggregate is a gem made up of microscopic to sub-microscopic crystals all intermeshed together. Examples are various chalcedonies and jade).
The
AGG Reaction :
All of the above is MUCH harder to put into words, or to read and comprehend, than it is to observe and recognize. Let's do some tests. In the picture below are three pieces of gem material. One is quartz, one is glass and one is chalcedony. We'll observe their reactions under the polariscope and see what we can conclude.
Items to test : One is Chalcedony, one Glass and one Quartz Specimen 1 :
The first piece to be tested is the colorless one on the upper left. See its reaction below: (Note that you can, by looking at its shape, determine which of the three pieces it is, and that it has been rotated approximately 90 degrees in the second picture.)
Specimen 1 : Under "crossed filters" in two positions Specimen 2 :
The next piece to be tested is the light purple one on the upper right.
Specimen 2 : Under "crossed filters" in two positions Specimen 3 :
The final piece to be tested is the light brown one on the bottom.
Specimen 3 : Under "crossed filters" in two positions Keeping in mind that glass is amorphous and therefore SR, quartz is DR and chalcedony is a aggregate, it is quite clear which is which.
Testing Refractive Index :
The refractive index (RI) of a gem is one of the most important characteristics determining its appearance, and is also a very useful piece of data for purposes of determining the species of an unidentified gem.
Without Equipment :
Using no equipment it is sometimes possible to determine an approximate range for the RI of a polished gem. This is because RI generally correlates physically with the density of a gem, and visually with its luster, brilliance and dispersion. So, if I find a gem which is heavy for its size, has a higher than vitreous luster, is extremely brilliant and shows dispersion, I can just about conclude that its RI will be found in the upper ranges for gems. On the other hand, a notably lightweight piece with sub-vitreous luster, little brilliance and no dispersion, is probably to be found near the lower end of the RI range. I say it is sometimes possible, though, because the degree of polish or lack of it, the number and types of inclusions, and the color or condition of the gem may make even a ballpark estimate difficult without some kind of equipment.
Using Liquids of Known RI :
You will recall from Lesson 3 how it is possible using liquids of known specific gravity to work out an approximate SG for a gem by immersing it and observing the reaction. There is a similar technique that can be used for RI. This technique called "Relative Refraction" uses liquids whose RIs are known. When a transparent gem is immersed in a (colorless) liquid, how clearly we can see details of the gem depends on the RI of the gem compared to the RI of the liquid. A critical concept here is "relief"; we say that a gem has high relief when it stands out as sharply visible in the liquid, and low relief when it doesn't. The greater the RI of the gem above that of the liquid in which it is immersed, the greater its relief.
1. A gem that is immersed in a liquid whose RI is well below its own shows high relief, the gem and its details are clearly visible
2. A gem that is immersed in a liquid whose RI is moderately to slightly below its own shows moderate to slight relief, the interior details and outline of the gem are harder to see.
3. A gem that is immersed in a liquid whose RI is the same as or higher than its own shows no relief, no edge, or interior detail is visible. The gem, if colored, looks simply like a hazy colored area in the liquid, and if it is colorless, it virtually seems to disappear!
Using this basic idea we can compare the relief of two different gems in the same liquid, or we can test the same gem in different liquids. Each technique would give us information about the relative refractive index of the test pieces.
Three common liquids used in this sort of testing are: water (RI = 1.33), a commercial compound called "Refractol" (RI = 1.56) and methylene iodide (RI = 1.74).
Liquid sold commericially for relative refraction testing: RI = 1.56 Before proceeding any further, let's see a demonstration: below is a bottle of "Refractol" and the gem petalite. The RI of petalite is between (1.50 - 1.51). Watch what happens :
Going, going, gone Notice the reflections and detail visible when the gem is in air, compared to when it is immersed in the liquid. In the Refractol, it seems to disappear. You can tell that there is something in the jaws of the tweezers, but you can't make out any detail.
This happened because the RI of the gem (1.50 - 1.51) was below that of the liquid (1.56). If I had not already known that the gem was petalite, but instead, was trying to identify an unknown, this test would have eliminated a great many possibilities.
Another reason why gems are sometimes immersed in such liquids is that by diminishing the relief and reflections, internal characteristics like color zoning, fractures and inclusions sometimes stand out more clearly.
If you ever go to a store or show where gem rough is sold, you can see potential purchasers dipping the rough into water the dealer has provided to get a better interior view (or less appealingly, licking the piece when no water is available!)
Using
a refractometer to test RI :
When no reading can be obtained (almost always because the RI of the gem is above 1.80, the reading is said to be "OTL", or over the limits), which, in itself, is often useful information for identification purposes.
Standard Refractometer This is a delicate and expensive piece of equipment which has numerous limitations, and is difficult to learn to use properly, yet it remains the single most useful tool for the gemologist doing gem identifications. Most models, like the one seen above, require a separate light source. The most important part of the device is a leaded glass "hemicylinder", seen as the slightly yellowish rectangle above, upon which the gem whose RI is to be read, is placed. A drop of very high RI "contact" fluid (methylene iodide saturated with dissolved sulfur, plus 18% tetraiodoethylene), is placed on the hemicylinder to assure that there is no air between it and the gem.
A system of internal mirrors reflects light which has been bent to a specific degree by the gem, so that it falls, as a shadow, upon a scale visible to the observer. All of this is based on firmly established principles of mathematics and optics that needn't concern us here.
RI Readings :
A refractometer that is ready to take a reading In the picture above, a light source is in position, the recently cleaned gem is table down on the hemicyclinder with a drop of contact fluid in place. The picture below shows what a such a reading might look like. The reading, shown is between 1.56 and 1.57. With practice it is possible to be quite accurate at estimating that third decimal place.
Refractometer reading Due to the
differential refraction of the various colors within white light,
readings obtained with white light (as seen above) are somewhat
fuzzy. Using a single wavelength source (usually yellow), produces
much sharper shadows. An SR gem will give the same RI reading
in all eight positions as it has no birefringence, but a DR gem
will give different RIs in different directions. By subtracting
the lowest from the highest, the gem's BR can be calculated.
You may recall from the topic of selective absorption, that gems may absorb parts of the visible light spectrum and convert them to heat. It is also the case that gems (due to the specifics of their chemical makeup or crystal lattices) may absorb other types of electromagnetic radiation (UV, Xrays) and convert them to visible light. This phenomenon is known as photoluminesence.
UV, or ultraviolet radiation is that part of the electromagnetic spectrum that has wavelengths just shorter than those of visible light. We divide the UV spectrum into two parts: longwave starting at 365 nm (LW), and shortwave starting at 254 nm (SW). Remembering that wavelength and energy content have an inverse relationship, this tells us that SW is the more energetic type. Although there are several expressions of the photoluminescence phenomenon that can be tested for in big gem labs, the type which is most useful to the average gemologist is fluorescence testing.
Fluorescence: When a gem absorbs either SW or LW UV, or both, and immediately emits visible light, the phenomenon is called fluorescence. In order to test for fluorescence it is necessary to have a controlled source of SW and/or LW and a darkened viewing chamber. (It is also prudent to have UV protective eyewear as exposure to these rays can be damaging.) The specifics of the color and intensity of fluorescence can sometimes be a useful diagnostic test in identifying gems.
A typical UV test lamp, as seen below, usually consists of a light source which produces the UV with a pair of filters covering it. On one side a filter blocks SW and permits LW to pass, and on the other end LW is blocked passing the SW. In the model below, a simple metal slider mechanism is moved from one side to the other to block out the undesired wavelengths. The test would be performed inside a viewing chamber that blocks out all visible light. The gem to be tested must be very clean as skin oil and dust particles often fluoresce brightly.
Fluoresence tester : set up to test with SW, set up to test with LW Fluoresence can be absent, in which case we say the gem is inert, or present in weak, moderate or strong form. The light emitted by fluorescence can be the same color or a different one from the color of the gem itself, and a gem can have the same or different reactions to LW and SW.
Fluorescing red under UV (no visible light is present) Fluorescence testing is usually, at best, supplemental, it is rarely defintive. There is one notable case, however, where the test can provide proof of identity: Benitoite. There is no other blue gemstone which shares its distinctive reaction of being inert to LW, and fluorescing a strong, chalky blue with SW.
Benitoite under: visible light, LW UV, SW UV Testing the Absorption Spectrum of a Gem :
A "spectroscope" contains a prism (or a diffraction grating) which serves to disperse incoming light. This light, which has been reflected or transmitted from the gem being tested, enters the spectroscope, and is dispersed to display as a rainbow spectrum through the eyepiece. If significant selective absorption has taken place, then certain portions of that spectrum will be "missing" or reduced. Black or darkened lines or bands indicate which wavelengths have been absorbed by the gem and to what degree. The lines can be very distinct and quite sharp indicating that the gem has absorbed very strongly in a small region of the spectrum, or broad and indistinct indicating a more general absorption over a wider band of wavelengths.
Hand held models are relatively inexpensive, but difficult to learn to use effectively, and to provide adequate lighting for. Desk models are very much more expensive (the one from GIA, pictured below, is $5000) but easier to use, and more accurate. The spectroscope is used with a handbook of printed gem spectra for comparison. Big labs have high tech versions, using specialized lighting (or other energy sources, like infrared or Xray), special cooling (liquid nitrogen), etc.
Procedure for using a handheld spectroscope
GIA "Prism 1000"model spectroscope By comparing the two spectra below, it is easy to see how distinctive an absorption spectrum can be. In such cases the spectrum may be diagnostic of species, coloring agent or even location of origin. Less than 25% of gem species, however, show clear, unambiguous, absorption spectra like these. The percentage of gemologists who can accurately use a pocket spectroscope is probably even smaller than that! (And I cannot be counted among them.) Usually, therefore, spectroscopic examination is a supplemental, rather than a diagnostic, test.
Zircon Spectrum
Almandite Garnet Spectrum |