| PHYSICAL PROPERITES OF GEMS Properties
of Gemstones :
There are two sets of characteristics possessed by every gemstone, and by which they are studied, identified and evaluated. 1. Physical properties and 2. Optical properties.
In this lesson we'll be concerned with physical properties : those which do not depend on the gem's interaction with light to be expressed or measured.
All properties of gems (whether physical or optical) derive from the underlying three dimensional structure and chemical composition of the gem. Or to put it another way, the chemical elements that make up the gem, and how the atoms of those elements are put together to create its inner structure, determine all those properties that we can see, feel, and measure.
Amorphous
vs Crystalline :
Crystalline gems have a specific chemical formula, and a well defined, highly predictable internal structure, known as a crystal lattice. Amorphous gem species also have a specific chemical formula, but their constituent atoms are not arranged in such regular and predictable patterns as those of crystalline materials.
Amorphous Gems :
"Amorphous" literally means "without form", but, of course, these materials have a form it's just not highly regular and predictable, nor is it expressed outwardly by the formation of crystals. Some examples of amorphous gems are: the natural glasses, amber, jet, opal, and "metamict" minerals.
Natural
Glasses :
Obsidian ranges in color from light yellow through brown to black and can be transparent, translucent, or opaque. Those of our ancestors, who lived in areas of volcanic activity, made ready use of these natural glasses.
Obsidian Artifacts In some cases, due to the presence of other minerals with different crystallization temperatures, when the molten material cools, crystal inclusions may be formed. These can give the obsidian an interesting pattern, or affect the structure in such as way as to cause an optical phenomenon, like iridescence. Although most obsidian is drab, single-color translucent material, two interesting and more showy forms of this volcanic glass can be seen below :
"Snowflake" obsidian
"velvet" obsidian
The most commonly seen tektite is a green, near transparent type found in the Moldau River Valley in Eastern Europe, known as Moldavite. An intriguing light yellow form of natural glass has been found in several areas within the Libyan Desert, and, to date, has not been associated with a meteor impact, so its origin remains uncertain.
Moldavite Cabochon
Faceted Moldavite in Jewelry
Libyan Desert Glass Amorphous Organics :
A number of organic gem materials have an amorphous structure. Species like amber and jet which are composed of organic molecules, (those of evergreen tree resin, and the wood of certain hardwood trees, respectively) which have been altered into a near "plastic" polymeric state by geologic forces and time, are examples.
Reverse-carved Amber Cabochon
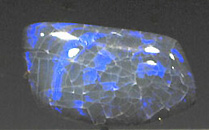
Jet Carving Opal :
Although opal is one of the most diverse gem species, with a large number of named varieties, all share a common structural feature. An electron-microscopic view of opal shows that it is made of row upon row of stacked silica spheres, the exact arrangement, pattern, and size of which determine the body color, transparency and degree of color play of the opal.
Opal forms as a colloidial solution of tiny silica particles in water, and as the water is lost the material solidifies to a microscopically porous, amorphous state.
Metamict Minerals :
Most zircon specimens that you will see, are crystalline gems, but a few pieces (generally a dull olive green color) have lost some, or all, of their crystalline structure, and have become disorganized internally to a glassy state. This transformation is due to the effects of radiation, and such a material is said to be "metamict".
The radiation source is usually from impurities within the zircon itself, but can be from surrounding rocks. This phenomenon can occur naturally in several minerals, but zircon, and perhaps ekanite, are the only ones of gem significance. These glass-like zircons (sometimes called "low" zircons) do not have the same super-bright luster and brilliance of the crystalline type, and are mainly sought as curiosities by collectors.
Metamict zircon Crystalline Gems :
The highly regular, and sometimes startlingly angular shape of some well formed crystals can seem eerily out of place in the world of Nature, with its more familiar curving and flowing lines. It's no wonder, then, that a rich history of mystical and mythological lore pre-dates, and coexists with, today's chemical and physical understanding of crystal structure.
Imagine the reaction of our ancestors, so used to the shapes and forms of flowing water, curling fire, gnarled tree branches, curving shell, roseate flowers and sinuous leaves, when they saw something that looks like the images below, perhaps in a mass of rock on a hillside, or upon cracking open an ordinary looking boulder.
Natural Pyrite Cubic Crystal in host rock
Amethyst Crystals inside a Geode The internal regularity that the outer features of such structures implies, was as evident to our ancestors as it is to us, but it was not until the beginnings of modern physics and chemistry (in the 17th - 18th centuries) that some of the underlying causes came to be understood. Full revelation of the most intimate details of crystal formation and properties awaits future generations, but major gains were made in the early 1950's with the advent of a technique called Xray diffraction.
Although definitely more"hi-tech", the principle behind this technique is essentially the same as used in an ordinary medical Xray machine. To illustrate: a beam of Xrays travels through your arm, let's say, to Xray sensitive film below. The dense tissue (bone) absorbs more of the Xrays than does the soft tissue, so the film is exposed differently, and a high contrast picture is made.
In the case of mineral crystals, the dense areas (where atoms are closer together) absorb more Xrays than the less dense ones (where atoms are further apart), and a high contrast picture is produced. For those who have been trained in reading such photos, inner details of crystal structure can be deduced by interpreting the patterns.
Single
Crystal vs Aggregate Gems :
The pyrite and amethysts, pictured above, are examples of single crystal gems. If, however, you can imagine shrinking the amethyst quartz crystals down to very, very tiny proportions and pushing them together in random orientations such that you'd need ultra-strong magnification to resolve them, you'd have an idea of the internal organization of an aggregate gem, like chalcedony (an aggregate form of quartz).
Both amethyst and chalcedony are the same species: namely quartz, so their crystals (whatever their size) are of the trigonal system, and their chemical formula is SiO2, but the difference in the crystal sizes and arrangement creates some notably different physical and optical properties in the two varieties. For example, amethyst, and other single crystal quartzes, are commonly transparent and one color, while chalcedonies, agates, and other aggregate quartzes are translucent to opaque and often have complex color patterns. Although single crystal and aggregate types of quartz are equally hard, the aggregates are notably tougher. Recall this pair of photos from Lesson 2 which also serve as good examples here :
Single crystal (amethyst)
Aggregate (agate) Single Crystal Gems :
Single crystals can be large truck size or even bigger. I recall with pleasure an undergraduate college geology field trip made to Crystal Cave in Put-in-Bay, South Bass Island, Ohio. The cave, which was actually an enormous underground geode, had walls and a ceiling composed of huge celestite (or celestine) crystals.
Celestite crystal cluster from Ohio, near Crystal Cave Single crystal gems grow in clusters or individually, and they can be formed within, or attached to, another mineral, or loose, as so-called "floater" crystals. Single crystals can be quite small, but they will still qualify as single crystals (not aggregates), as long as they are large enough to be visible as separate entities without high magnification. "Drusy" gems consist of such small, to tiny, single crystals, which have grown upon a matrix.
Single Hanksite crystal
Quartz "floater" Crystal Cluster
Spinel Crystal in Calcite Marble Matrix
Aggregate Gems: Micro- vs Crypto-crystalline :
Microcrystalline
aggregates :
Jadeite Jade
Nephrite Jade Cryptocrystalline
aggregates :
Agate
Jasper
Chalcedony Gem
Rocks :
Unakite
lapis lazuli
Chinese writing stone Crystallograpy
:
The Crystal Systems :
Scientific analysis has determined that there are seven basic plans upon which all mineral crystals are built they are known as the "crystal systems". Each of the systems has a unique architecture, based on the lengths, and angles of intersection, of planes through the crystal called "axes", about which there are degrees of symmetry.
Crytstal systems figure Unit Cells :
The innermost structure of each crystal is based upon atomic-scale building blocks that exhibit the symmetries shown in the "axes" column in the diagram above. These tiny building blocks are called "unit cells". The shape of a unit cell is different in each of the crystal systems: a cube in the cubic system, a "brick" for the tetragonal system, etc. These tiny structures assemble themselves as the crystal grows, and build the crystal up to its finished size and shape. It might seem, from the diagram above, that there are only a few outward forms (or "habits") possible, given the seven types of unit cells available but in the real world, we find that mineral crystals come in nearly an infinite set of shapes and sizes. How can this be?.
Because it is easiest to visualize, I'll use the cubic (also known as isometric) system to illustrate. The unit cell in this system is a cube: picture a baby's building block set, or (if you live here in Nevada), dice.
Is it possible to build a big cube out of little cubes?... Sure, just stack them up 5 x 5 x 5 or in any other equal dimenisons, and your many little cubes become one big one. Such is the mechanism by which the impressive pyrite cube seen earlier in the lesson was built from the little, cube shaped, unit cells of the mineral pyrite. It shouldn't surprise you, then, to learn that diamond (which is also a member of the cubic crystal system) is sometimes found in natural cubes.
Natural diamond crystals
But
using those same blocks or dice, can you build a pyramid?... You
bet! Start with a square base, and decrease each square layer
uniformly until to get to the top single cube. (5 x 5, then 4
x 4, then 3 x 3, etc). Look at the second of the "typical
forms" for the cubic system shown in the diagram above. Can
you see it as two pyramids attached to each other, base to base?
That shape is called an octahedron (meaning eight sides) and it's
a common form seen in the crystals of gems of the cubic system.
(Why are the faces of the octahedra so smooth? because the cubic
unit cells are really, really tiny, and there are enormous numbers
of them.
Fluorite
Spinel
Octahedra Characteristic crystal forms such as those above, that are easily recognized, and are typical of a particular mineral, are known as its crystal "habits", but no gem species is limited to these ideal shapes. You can also see, I'm sure, that it's quite possible to build a random looking structure out of your blocks or dice, one that has no readily categorizable outer shape. This frequently seen habit in crystalline gems is referred to simply as "massive".
Now, recognizing that each of the seven crystal systems has a different unit cell, and that these unit cells can be put together in many, many ways, is there any wonder that the diversity of crystal forms in Nature is staggering, and such a challenge, and delight to mineral specimen collectors?
A few of the crystal habits, due to their similarity to common objects, are especially recognizable and have acquired special names, as demonstrated by the specimens below :
Acicular (needle-like): golden rutile crystals in quartz'
"puffball-like"mesolite specimen of radiating acicular needles
Prismatic (pencil-like) Tourmaline Crystals
Red Beryl Crystal in Matrix
Quartz with Black Manganese Dioxide Crystal Inclusions
Dendritic Native Copper Crytals
Rainbow Pyrite Crystals on Matrix
Botryoidal (seen in aggregate gems only, like a bunch of grapes or bubbles) : Blue Chalcedony
Factors affecting crystal appearance :
Although the crystal system and unit cell which are characterisitic of a particular gem species set certain parameters in regards to their formation, there are also a mulitude of environmental factors that will determine precisely what size, shape, color, and clarity a particular crystal will have. (We'll be taking a look at how different species of gems are formed in Lesson 10, "Gem Formation", but regardless of the specifics, all gem formation processes are affected by the same factors listed below).
Temperature/Pressure :
The effect of rapid versus slow cooling of a melted material has already been alluded to, but there is more to the story. The same molten mass of atoms, or the same solution, or vapor of materials can crystallize differently, depending on the temperature and pressure at the time, and in the place, where crystallization occurs. This is because there can be more than one stable crystal lattice composed of the same atoms. The various stable configurations that a particular gem species can crystallize in, are referred to as its "polymorphs".
Polymorphs :
When two materials have the same chemical formula but have crystallized differently (due to each being subjected to different temperature/pressure conditions at formation), they are called polymorphs. The most famous examples are diamond and graphite. Both have the same chemical formula (just C, pure carbon), but the "lead" in your pencil and the diamond on your finger, obviously exhibit quite different properties. Graphite crystals are formed of sheets of tightly bonded carbons atoms in layers which are very loosely bound to each other, allowing lots of slipping and sliding. Diamond crystals have each carbon atom bonded tightly to four others surrounding it in all directions, so the whole structure is very strong and durable.
Single Crystal Graphite and Diamond (uncut dodecahedral (12 sided) crystal)
Polymorphs of Carbon
Kyanite
Quartz from Madagascar with fluorite crystal inclusions, inset picture at 10x Special Growth Phenomena :
Twinning :
When growing crystals of the same mineral share one or more faces, the result is a crystal "twin". Depending on the nature of the twinning, which can be on either a visible, or a microscopic scale, the shape of the crystal might be dramatically affected, or the material's properties could be noticeably altered. Sometimes, evidence of twinning can be seen in a crystal or cut gem due to unusual color or inclusion patterns.
Twinned Quartz Crystals in "rabbit ear" form
Twinned Octahedral Diamond Crystals which form a characteristic flattened triangular "maacle"
Rare "hour glass" Twinned Gypsum Cyrstal from Australia
lepidocrosite platelets of the cabochon
Alternating color sectors of the Crystal slice Phantoms and Negative Crystals :
Due to changes in environmental conditions, starts and stops of crystal growth occur. When other minerals, which are favored in the new conditions, start to grow, they sometimes crystallize on the "old" faces of the temporarily inert material. When conditions change, and the host once again starts its growth, evidence of the pauses may now be visibly captured as outlines of the temporary stopping points, called "phantoms".
Likewise, certain conditions may completely block the growth of an interior portion of a crystal leaving a void which is bounded by the sides of the crystal around it at first glance this "negative" crystal looks like a solid crystal inclusion, but it is indeed empty.
Hematite Phantoms in Calcite
Negative Crystal in Quartz Seudomorphs
:
Copper ps. after Aragonite In
the first picture above you see what appears to be a twinned pyrite
crystal, but chemically and physically it tests not as pyrite, but
as Goethite, the second picture shows what appears to be a hexagonal
crystal, but it is made entirely of the cubic system mineral, copper.
Pseudomorphs occur when environmental conditions occur that cause
the replacement of one chemical compound with another without altering
the pre-existing three dimensional structure. Mineralogically, the
item is named as an "X" pseudomorph (ps.) after "Y".
(Goethite pseudomorph after pyrite, for example. ) Likewise those
"petrified" fossils spoken of in Lesson 1 are more technically
known as "chalcedony pseudomorphs after bone", or "opal
pseudomorphs after wood".
In addition to categorizing gems by their three dimensional structures, we can also view them as belonging to various chemical groups. Due to their related chemistries, some quite different looking gems share some of their basic properties, while other gems which look rather similar, differ markedly, due to their unlike chemistries.
Even if you haven't yet taken a college chemistry course, you are undoubtedly familiar with the basic idea that the material world around us is made of up of units (atoms) of unique substances called elements. Examples of elements would be carbon and aluminum. These elements are held together in crystal lattices, molecules and amorphous materials, by attractive forces called chemical bonds.
In the world of minerals, certain groupings occur quite commonly. For example, oxygen frequently occurs bonded to atoms of a metal (like iron or aluminum). We call such compounds oxides, and oxide gems have some characteristics in common. There are dozens of chemical groups which could be listed if all gem species were taken into account, but in this course, you will be required to recognize, and recall, only five major groups, the: silicates, oxides, carbonates, phosphates and native elements.
Accounting for nearly 60% of gem species, silicates are the most important group, closely followed in prominence by the oxides. These two groups have in common, that their member species tend to be relatively hard and stable, while the carbonates and phosphates are generally softer, and susceptible to attack by acids.
Recognizing which chemical group a species belongs to is simple, as long as you know what to look for.
Here's the first one for you : looking up emerald on page 152 you see : Be3Al2(SiO3)6
Emerald
Amethyst
Tigers Eye
Moonstone
Rhodolite Garnet
Blue Topaz
Bi-colored Tourmaline
White Zircon
Ruby
Spinel
Malachite
Calcite
Azurite
Turquoise
Apatite
Gold in Quartz necklace
Platinum Nugget
Diamonds Two interesting native element examples, not used as gems, but often sought by collectors are sulfur and mercury. Pure sulfur occurs in bright yellow crystals which would tempt the faceter if they were not so heat sensitive that just holding them in the hand causes them to crack, and rare native mercury has the distinction of being the only metal found in a liquid form at normal ambient temperatures.
Crystals of pure Sulfur from Mexico : beautiful to look at but too fragile to touch
Droplets of liquid native Mercury in matrix rock from California Major Physical Properties :
Although there are a dozen or more physical properties which can be measured, in this course we will concentrate on just a few. In particular, our focus will be on those which are either visible directly, or measurable with minimal equipment, and those which are most important as indicators of a gem's identity, and/or its suitability for particular uses :
Cleavage :
Since cleavage, or lack of it, is a species trait, it also serves as a good gem identification criterion. In the examples below, the number and completeness of cleavage of three species are shown.
Species with easy or perfect cleavage, particularly when such is the case in multiple directions, are poor risks for most jewelry applications. Not all gems show cleavage however, for example tourmalines, sapphires, and garnets do not.
Apatite : Two imperfect (note that cleaved surfaces are somewhat rounded and irregular)
Spodumene : Two perfect (note extremely flat, smooth breaks)
Fluorite : Four, Perfect Miners have long used the cleavage properties of gems in trimming the stones they find. "Cobbing" is the act of smacking a piece of rough sharply and precisely with a hammer to break off any unstable (already partially cleaved), or included areas. Knowledge of the cleavage planes in the material being mined is essential to efficient use of this technique.
The use of cleavage is perhaps most well known in diamond cutting. We've all seen photos or videos of that tense moment when the diamond cutter inserts the wedge at a particular spot on the diamond and strikes it with a mallet. If all goes well, the stone splits precisely where the cutter wanted, and expected, it to. It is said that the expert that first cleaved the (up to that time) largest rough diamond ever found (The Cullinan) had studied it for months to determine its cleavage planes, and upon striking the first blow fainted dead away from anxiety. All was well, however.
Fracture
:
Playing on the resemblances of certain fracture types to well known surfaces and objects, terms like conchoidal (shell-like), splintery, uneven, step-like, and granular are used. Like cleavage, this is a species specific characteristic which has value in the identification of gems.
Citrine Quartz: Conchoidal
Charoite : Splintery
Turquoise : Granular
Coral : Uneven Conchoidal
fracture is the most common, and is found in corundum, beryls, all
the quartzes, opals, and both natural and man-made glasses. Turquoise
and coral are commonly simulated by glass.
The concept of gem durability was introduced and described as being made up of hardness, toughness and stability. Let's now look at each of these factors in more detail.
Hardness :
The
tendency to resist scratching in a gem is known as its hardness.
Of the three factors comprising durability, it is the most familiar.
Even those folks with just a passing interest in gems know that
they can be ranked on a scale of hardness. Hardness is primarily
the result of the strength of the chemical bonds between the gem's
constituent atoms (how tightly they are bound to one another).
The familiar 1-10 Mohs' Scale of hardness, is not an absolute measure, but rather a relative one a kind of "pecking order". Gems ranked at a higher number on the scale can scratch those ranked lower, and will in turn, be scratched by those whose number is higher than theirs.
Frederich Mohs, a 19th century German mineralogist was the originator, and we still use his scale, with the minerals which he designated as reference points today. For example, (softest) talc = 1, quartz = 7 and diamond = 10 (hardest).
Talc, the softest on the Mohs' scale
In mineralogy, one of the key tests commonly used for purposes of identification is a "scratch" test, which is done with a set of implements known as hardness points. These, usually steel, "pencils" are tipped with various minerals (or metals) of known hardness. By drawing them across the surface of an unknown mineral sequentially, the tester can determine the sample's approximate hardness. In gemology, such tests are rarely used as they are destructive in nature. Exceptions might be in testing the bottom of a carving, or a piece of gem rough, or a bit of material which has broken off. Another drawback of the standard hardness points is that they are not precise, but limited to giving a "ballpark" estimate.
In a laboratory setting, exquisitely precise measurements can be made with sclerometers. These devices use diamond-tipped, hydraulically operated probes, and can give an absolute reading on the force necessary to penetrate the surface of a material.
Not many hikers, nature lovers, or rockhounds carry hardness points with around with them on their treks, but the use of just a few ordinary materials can allow such individuals to do pretty good hardness tests in the field.
The Practical or Field Mohs' Scale :
1-2 : easily scratched by fingernail
3-4 : scratched by copper coin
5-6 : easily, and not so easily, scratched with pocket knife
7 : scratches window glass/scratched by steel file
8-10 : scratches window glass, but not scratched by steel file
Hardness can be directional. This is actually quite understandable, as it depends on chemical bonds which can differ in strength, and in distance from each other, depending on which axis of the crystal we are observing. Generally such differences are relatively small and of litttle consequence, but there are two notable cases where they are dramatic and important. 1) Kyanite is notoriously difficult to cut because of its extreme directional hardness differences. 2) Diamond cutting would scarcely be possible unless the cutters could use the directional hardness of that gem to their advantage.
Soft Gems :
Ivory and Jet : 2.5
Pearl : 3
Fluorite : 4 Gems of intermediate hardness :
Scapolite : 6
Tanzanite : 6.5
Garnet : 7 - 7.5 depending on species
Tourmaline : 7.5 Hard Gems :
Spinel : 8
Topaz : 6.5
Chrysoberyl : 8.5
Sapphire : 9 Toughness
:
All other factors being equal, the harder the gem, the tougher it will be, but all other factors are not always equal. Take the case of topaz, for example. At hardness 8 it seems to be a pretty rugged gem, but if we consider its strong tendency to cleave in one direction, in reality, it is rather fragile.
Likewise, diamond, the "star" of the hardness game, is only ranked as "good" when it comes to toughness because of its cleavage and fracture potential. Diamonds are usually cut with a flat culet facet at the tip of their pavilion, rather than coming to a sharp point as do colored stones. This is due to the likelihood of a fracture (or cleavage) in the fragile culet zone. When purchasing a diamond it is a good idea to check the girdle under magnification to make sure that it is not excessively thin, as this is another site of special vulnerability. Likewise, the corners and points of cuts like baguettes, trillions and marquis are vulnerable, and should be protected by the mounting when used in jewelry.
Fracture on the Girdle of a Diamond
Cleavages on Diamond with classic "staircase" pattern On
the other hand, nephrite jade with its hardness of 6.5 might seem
to be delicate, but due to the felted, fibrous nature of its aggregate
crystals, it is literally the toughest gem on Earth! So it is with
pearls, which with their extremely low hardness, would barely be
wearable at all, except for their moderately good toughness. It
results from the layered, overlapping nature of the aragonite mineral
plates of which the pearls are made, and the proteinaceous "mortar"
that holds these brick-like layers together.
There is no numeric scale on which toughness is measured, rather, relative terms such as: exceptional, excellent, good, fair and poor are used.
Fragile Gems :
Topaz
Sunstone
Sodalite
Serpentine Gems of intermediate toughness :
Tourmaline
Iolite : fair
Chrysoprase
Diamond : good Tough Gems :
Sapphire
Hematite : excellent
Jadeite Jade
Nephrite Jade : exceptional Stability :
Stability in a gem is a measure of its ability to resist changes due to exposure to light, heat and/or chemicals. Not only does stability affect wearability, but it also dictates appropriate ways of fashioning, cleaning and storing the gems. Most gems are stable, but a few (even some quite popular ones) are unstable, and must be handled accordingly.
The Effects of Heat :
Dehydration :
Badly crazed Opal It is sometimes suggested that opal gems and jewelry items be stored in water or oil when not being worn this is NOT good advice. Water will not hurt the opal, but it will not help it either. The type of "chemically linked" water that is lost when crazing occurs cannot be replaced by soaking, nor can this procedure be used as a preventative.
It is the structural details of the particular type of opal, including the percentage of water in it, that determine the likelihood of crazing. Reputable opal dealers "proof" their material before it is sold, by subjecting it to hot dry conditions for months. Generally, those pieces that survive such treatment will be stable under normal wearing conditions.
(Leaving an opal on a car dashboard for hours in the August sun, or forgetting that your opal ring is in your pants pocket, and then putting the pants in the dryer for an hour on high, would NOT be examples of normal wearing conditions! Soaking in oil is an especially bad idea as opal is a porous gem and the oil seeps inside and then discolors over time, degrading the gem's beauty.)
Thermal
Expansion :
Other gems, such as apatite, expand so rapidly with sharp rise in temperature, that their crystal structure is damaged, and they crack or even shatter. Heat sensitivity of that degree makes it very important for lapidaries cutting such gems, and jewelers working on mountings containing them, to keep the gem cool during these processes.
The
Effect of Inclusions :
To an extent, heat treaters can ameliorate such effects by very, very, slowly raising and lowering temperatures. Tanzanite heaters might take 12 to 24 hours to incrementally reach the desired temperature, hold the gems there for several hours, and then take another 12 to 24 hours to gradually cool them down. At the highest temperature levels, though, such as those required to heat treat corundum, or those used for "color diffusion" processes, nothing can prevent heat damage. This is good news in a sense, though, because such internal and external cues to the heating, help the jeweler or gemologist spot the gem as one which has been subjected to extreme temperatures.
In the center of this picture of the interior of a gem under high magnification, you see an included, heat shattered crystal, broken into four pieces, and a series of stress fractures surrounding it positive evidence of high heat treatment in this gem There are cases where thermal expansion characteristics of gems are used to deliberately induce cracks or stress fractures. Pieces of amber which have been heated, and then quickly cooled, develop disk-like stress fractures called "sun spangles" which some consider to be attractive.
"Sun Spangles", stress fractures in heated Amber A very old method of dyeing gems, which is still occasionally used today, is called "quench crackling" single crystal gems, like quartz, for example, which would ordinarily not absorb dye are heated and plunged in cold water to fill them with cracks that, then, can take up the dye, giving apparent color to the whole piece.
Other Environmental Factors :
Light
:
Chemicals
:
Most gems in the unstable category, however; are sensitive more in virtue of their porosity, than because of their chemical makeup. Pearls and turquoise are two gems well known for their propensity to absorb cosmetics, perfumes, body oils, sweat, etc., and to dull and discolor as a result. Often fine turquoise gems are given a final polish with a layer of colorless paraffin wax to help seal and protect them from such degradation.
Lightly wiping chemically sensitive gems with a damp cloth after each wearing will help to keep them in good shape. Any gem which is suspected, or known, to be chemical or heat sensitive should be protected from steam or solvent cleaning methods. Such considerations also become a factor in gemological testing in that, turquoise, for example, cannot be placed in the chemicals that would be used to determine specific gravity, or those used in relative refractive index testing.
Unstable Gems :
Apatite and Opal : heat sensitive
Mexican Brown Topaz : fades in light
Turquoise : porous and likely to discolor with exposure to various materials Specific Gravity :
Specific gravity, also known as relative density, differs widely among gemstones, and is one of their most important physical characteristics from the viewpoint of gem identification. Specific gravity (SG) is the ratio of the weight of one unit volume of the gem to the weight of the same unit of water. For example, to say sapphire (corundum) has SG = 4.0, means precisely that a cubic inch of sapphire weighs four times as much as a cubic inch of water. In natural gems, SG values range from just over 1 (1.08 for amber) to just short of 7 (6.95 for cassiterite).
Light Gems SG < 3.O :
Amber : 1.08
Shell : 1.30
Meerschaum : 1.50
Opal : 2.10 Medium Density Gems : SG : 3 - 4 :
Andalusite : 3.16
Jadeite : 3.33
Chrysoberyl : 3.71
Sapphire : 4.00 Heavy Gems SG>4 :
Zircon : 4.69
Scheelite : 6.10
Anglesite : 6.35
Cassiterite : 6.95 A curious student might ask at this point : "Why do specific gravities differ so much?" The answer, satisfyingly, goes back to the basic premise of this lesson (that all physical properties of gems are the result of their chemical and structural makeup).
The various elements of which gems are made have atoms of different weights. Atoms of gaseous elements like hydrogen and oxygen are light, while metallic elements like aluminum and iron have heavy atoms. Chemists use "atomic weights" to describe elements rounding them off, here are some examples: hydrogen = 1, carbon = 12, oxygen = 16, aluminum = 27, silicon = 28, calcium = 40, iron = 56, zinc = 65, and lead = 207.
It's quite logical, then, that a cubic inch block of lead is going to weigh much more than a cubic inch block of aluminum. Extending that idea to gems, we can see that if a gem is made of relatively heavy elements it will have a greater SG than if it is made of lighter ones.
There is a second factor to consider, however; which is the structure : How are those atoms put together? Are they tightly packed or loosely arrayed? The examples below will help to illustrate the interplay of chemical make-up and crystal structure in determining specific gravity.
1. First, let's look at the case where structure is held constant but atomic makeup is different. Here, we'll compare two minerals that have the same crystal structure, in this case they both are of the orthorhombic system, and, they have identical chemical formulas except for substitution of one element for another.
Calcite : CaCO3 V.S. Smithsonite : ZnCO3
Both consist of five atoms per unit: either a Ca or a Zn plus one carbon and three oxygens. Both are put together with the "atomic packing" characteristic of the orthorhombic system of crystals. Their SGs differ though, with calcite = 2.71 and Smithsonite = 4.35.
Looking at the list above and seeing that calcium's atomic weight is 40 and that of zinc is 65 gives us our answer.
2. Now to examine the effect of structure, by holding the chemical makeup constant... Remembering the concept of "polymorphs" from the first part of this lesson, we'll compare calcite and aragonite. Both have the same chemical formula, CaCO3 :
Calcite : orthorhombic crystal system -vs- aragonite : trigonal crystal system
Both are made up of the same elements in the same proportions, but those building blocks are put together differently so their SGs differ, with calcite = 2.71 and aragonite = 2.94
Measuring Specific Gravity :
Although SG measurements can be made on either rough or cut gems, the gems must be unmounted, and composed of a single material. You cannot do a SG measurement on a gem that is set in a piece of jewelry, or on an assembled stone, like a doublet. Porous gems cannot be measured with at least two of the techniques, as the liquid they absorb affects the SG measurement, and, in some cases, can harm the stone. Detailed reference books meant for mineralogists or gemologists will list SGs for gems as a range, rather than a single number, due to the fact that individual specimens will differ slightly based on the number and type of their inclusions. (Your texts, meant for non-professional use, however, use a single number average of the SG range for the gem species). There are several ways in which SG is measured, and they differ in precision as well as suitability to different gems and circumstances.
Hefting
:
Heavy
Liquids :
Heavy Liquids Testing Set, SGs of liquids are printed on bottles, dropper bottles are for calibration To give a simple example, consider an unknown gem that floats quickly in the 3.05 bottle, sinks rapidly in th 2.57 bottle, and floats and sinks very slowly in the 2.67 and 2.62 bottles, respectively. That would tell you that the SG was between 2.67 and 2.62 and would allow you to rule out a great many minerals and focus any further tests on a smaller group of "possibles". Corundum (SG = 4.0) would behave quite differently from these observations, and could be excluded, while quartz, whose SG is 2.65 would behave precisely as described, and could not, therefore, be excluded.
Hydrostatic
Weighing :
SG calculation : Weight of gem in air divided by the difference between the weight in air and the weight in water, or : SG = Wa/ Wa-Ww
Hydrostatic weighing set-up consisting of an electronic balance with a special hanging basket apparatus in which the gem can be suspended in water without putting weight on the scale Again,
an example. We have an unknown gem whose weight in air is 5.10
ct and whose weight in water = 3.20 ct. The difference in the
air and water weights is 1.90 ct. Using the formula: SG = 5.10
ct/1.90 ct = 2.68. Looking in the tables at the back of the Hall
book we quickly find several gem possibilities close to that SG:
quartz (2.65), coral (2.68), aquamarine (2.69), and scapolite
(2.70). More importantly, than what it might be, a SG of 2.65
rules out a large number of possibilities that it cannot be. The
gemologist, like other scientists, progresses most often by weeding
out wrong hypotheses (as opposed to proving right ones!).
Is it?: Emerald (SG = 2.72 +.18/-.05); Tourmaline (SG = 3.06 +.20/-.06); Chrome Diopside (SG = 3.29 +.11/-.07) or Tsavorite Garnet (SG = 3.61 +.12/-.04) Hydrostatic
Test :
The
weight in air is: 2.420 ct.
Step Two : Assemble hydrostatic weighing chamber and "tare" balance.
The beaker with water is actually suspended by an arm off to the side and does not put weight on the balance pan, the plastic ring which holds a little metal basket for the gem, does put weight on the balance, though. Once everything is set up, we "tare" the balance (resetting it to zero) so that it ignores the weight of the plastic ring and gem basket. Now we are ready to place the gem in the basket where it will be weighed underwater. Step Three : Weigh Gem in water.
The weight of the gem in water is: 1.615 ct. The difference between the weight in air and weight in water is: 2.420 ct - 1.615 ct = 0.805 ct Step
Four : Calculate SG.
SG = 2.420 ct /0.805 ct = 3.01
Can we eliminate any possibilites? Check the SG range of each of the four possibilities. (Assume we have made accurate measurements and our arithmetic is correct).
Emerald (SG = 2.72 +.18/-.05); Tourmaline (SG = 3.06 +.20/-.06); Chrome Diopside (SG = 3.29 +.11/-.07) or Tsavorite Garnet (SG = 3.61 +.12/-.04)
The only gem whose SG range does not exclude 3.01 is: Tourmaline! The others are either too high or too low to qualify.
Testing by Heavy Liquids : Below are the results of the same test on the green gem, done with the set of heavy liquids :
3.32 : gem floats rapidly
3.05 : gem floats very slowly
2.67 : gem sinks
2.62 : gem sinks rapidly
2.52 : gem sinks very rapidly
Based on these results, the conclusion we must draw is that the SG is below 3.05, and above 2.67 (but closer to 3.05). If this were the only available testing method, we would be able to eliminate the chrome diopside and the Tsavorite garnet, but we'd have to do some other tests to discriminate between emerald and tourmaline.
Most gemologists prefer to use the hydrostatic method, not only because of its greater precision, but also because the heavy liquids smell very bad, and have hazardous properties such that gloves and masks must be worn when using them.
Home made heavy liquid test for Amber :
A saturated solution of salt water with amber and plastic immersed One
fun, and safe, heavy liquid test that can be done at home uses a
saturated saltwalter solution. (Make this by dissolving as much
salt in room temperature distilled water as it will hold). The SG
of this mini "Salt Lake" is about 1.13. Most types of
natural amber will float in it (SG = 1.08) while nearly all the
plastic materials used to make imitations of amber will sink as
their SGs are higher than 1.13. Imitation amber is rampant in the
gem marketplace (even in some of the better stores), so this is
a handy trick to know.
1. Magnetism :
Natural hematite is mildly to moderately attracted to a magnet, but an imitation version is so strongly magnetic that the difference is obvious.
2. Thermal Reaction :
Although, technically destructive, this test can usually be done on a very small, inconspicuous spot. Resin, lacquer and wax coatings on gems can likewise be detected as they melt or char under the hot point. In this case, the reaction is best observed under magnification, with the hot probe not touching the surface, but just barely above it.
3. Thermal Conductivity :
A few years ago, two developments occured which have all but made these devices obsolete. 1) A new diamond simulant, called Moissanite whose thermal conductivity is close enough to diamond to pass the test, has come on the market, and 2) synthetic diamonds are now becoming a common enough to be concerned about. Man-made diamonds which have the same physical properties as the natural gems, would, of course, pass the test as diamond.
4. Electrical Conductivity :
These machines have two systems, a thermal conductivity test, to first separate diamond and Moissanite from all other gems, then an electrical conductivity test to do the final separation should the thermal test indicate diamond. (Again, synthetic diamonds cannot be separated from natural ones with any basic physical tests).
Placing the probe on a gem initiates a thermal conductivity test to reveal CZ or other non-diamond simulants, then if the stone passes that, an electrical conductivity test follows to determine if it is diamond or Moissanite. |