| ELECTROMAGNETIC SPECTRUM
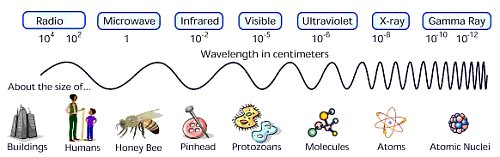
When we look at the world around us we are seeing visible light waves (or visible radiation). However, there are many other forms of radiation that we cannot see with our eyes. These types include gamma rays, x-rays, ultraviolet, infrared, microwaves and radio waves. Together with visible light, all these types of radiation make up what we call the electromagnetic spectrum - the complete spectrum of radiation. Light (or radiation) is made up of vibrating waves of electrical and magnetic fields. This is where the term electromagnetic radiation comes from. Electromagnetic radiation travels in waves which have different wavelengths, energies and frequencies.
Wavelength and Frequency :
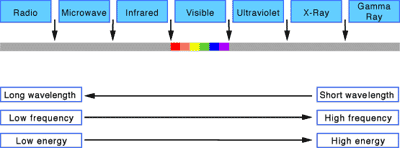
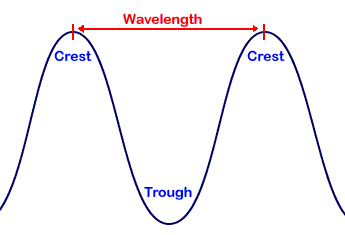
The wavelength is the distance between individual waves (e.g. from one peak to another). The wavelengths of visible light range between 400 to 700 billionths of a meter. But the entire electromagnetic spectrum extends from one billionth of a meter (for gamma rays) to meters (for some radio waves). The frequency is the number of waves which pass a point in space each second. Visible light frequencies range between 430 trillion waves per second (red) and 750 trillion waves per second (violet). The entire electromagnetic spectrum has frequencies between less than 1 billion waves per second (radio) and greater than 3 billion billion waves per second (gamma rays). Light waves are waves of energy and the amount of energy in a wave is proportional to its frequency. Wavelength increases, while frequency and energy decreases as we go from gamma rays to radio waves.
All electromagnetic radiation travels at the speed of light (186,000 miles or 300,000,000 meters per second in a vacuum). Objects in space send out electromagnetic radiation at all wavelengths - from gamma rays to radio waves. Each type of radiation (or light) brings us unique information so, to get a complete picture of the Universe, we need to study it in all of its light, using each part of the electromagnetic spectrum! Almost everything we know about the Universe comes from the study of the electromagnetic radiation emitted or reflected by objects in space.
The Electromagnetic Spectrum and the Color Light Spectrum :
The electromagnetic spectrum contains all of the colors of light that we can and cannot see from near DC to gamma radiation frequencies or wavelengths.
"Radiation, is that nuclear radiation?"
Before we address this question, we need to understand what the term "radiation" means. Radiation is "light" or waves (like radio waves) that, once generated by a source, can exist even when the source is turned off. Once generated, one can think of radiation as being pure energy propagating through space. Therefore, we can classify nuclear radiation, light, radio waves, and X-rays as examples of radiation. Now to answer your question, it turns out that the by-products of nuclear reactions radiate energy in the gamma radiation portion of the spectrum. The measure of units on the electromagnetic spectrum can be expressed in terms of frequency in Hertz (Hz), wavelengths in meters, energy in either Joules or eV, and/or temperature in degrees Kelvin.
This is confusing, why do we have so many different types of units all to mean the same thing ?
Scientist and engineers choose units base on convenience. For example, it is easier to request one gallon of milk than to ask for an equivalent of 3,786 ml of milk. Typically, power and radio wave engineers and scientists work with DC up through the radio wave spectrum and use the terminology of frequency. It is not uncommon for some radio wave engineers such as antenna designers to use both frequency and wavelength units. Microwave engineers and scientist use a mixed terminology of both frequency and wavelength. Optical engineers and scientist working in the infrared to ultraviolet portion of the spectrum use mixed units of both wavelength and energy (eV). Engineers and scientists that work in the x-ray and higher portion of the spectrum typically use the energy set of units, eV. A gamma ray may be as large as 10 sextillion Hertz.
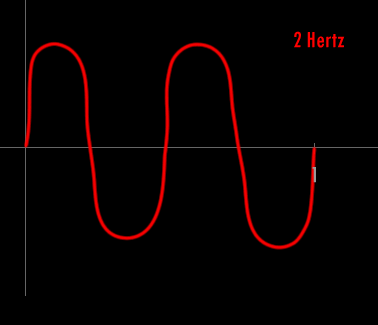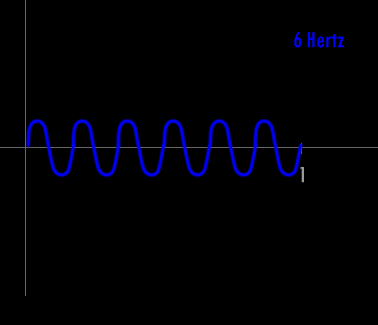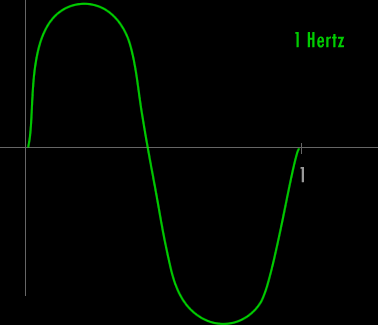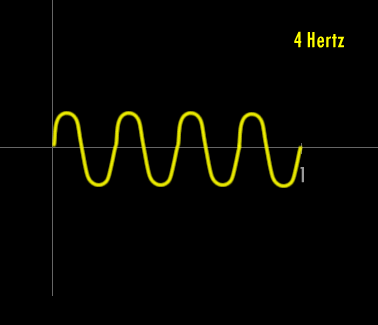
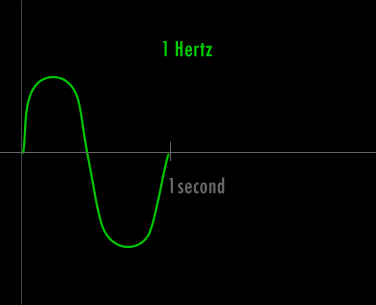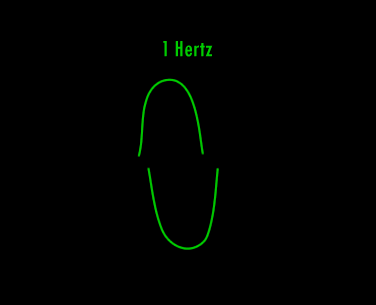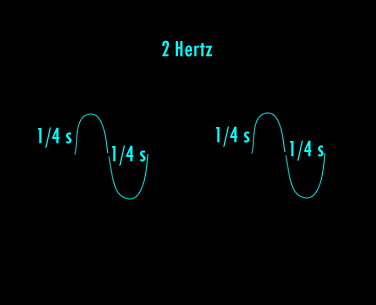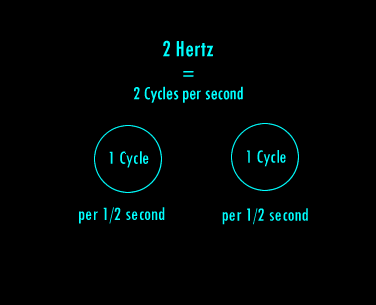
Now - what is a Hertz and what is a wavelength ?
A Hertz can be defined as 1 cycle per second. A cycle is one over the time it takes for the entire pattern of a periodic signal to repeat its nature. In order to think about this correctly, you must imagine yourself fixed in space with your measuring device to detect the electromagnetic wave (like an antenna) and a watch to record time. As the wave passes you, you record the time in seconds that it takes for the wave to repeat its nature. One over this time is the frequency in Hertz. Almost universally, engineers and scientists use the symbol f to represent frequency in Hertz. Various numbers of Hertz are graphed below where the horizontal axis is the time axis in seconds.
A cycle is one full revolution of a wave, so to speak. Kinda like one full revolution around a circle or 360 degrees or 2p radians. See the picture below to understand a cycle better.
Now you’re catching on. Well, wavelength is a lot like frequency except it deals with space. To understand this concept properly, imagine that you took a snapshot of the wave in space. At that instant, time is stopped in the film or photograph. Now, jump into the picture with a ruler and an imaginary detector that “sees” the pattern of the electromagnetic wave in the film or photograph. One now measures the spatial distance in which the wave repeats its nature. This spatial distance in meters is the wavelength. Almost universally, engineers and scientist use the Greek symbol l (lower case lambda) to represent wavelength. Can you imagine how the figures above may be changed in order to visualize the wavelength? Good for you, the time axis is changed to a space axis and Hertz is changed to meters.
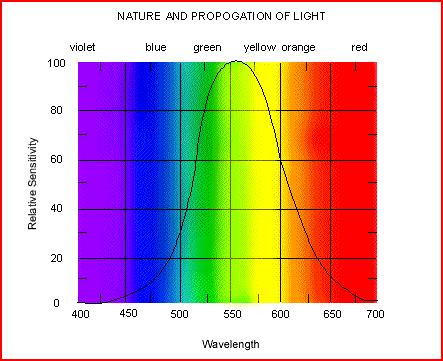
What colors do your eyes see? :
Red...Orange...Yellow...Green...Blue...Violet and all that is in-between.
The electromagnetic spectrum may be thought of as being composed of a complete spectrum of colors of pure energy or pure radiation. The color of the energy is determined by its frequency or equivalently its wavenumber, energy, or temperature. As indicate earlier our eyes can perceive only a small range of colors. A breakdown of the common radio wave and microwave bands are provided as well. Einstein, a great human philosopher, stated that nothing real can travel faster than the speed of light in vacuum. All scientists and engineers symbolize the speed of light with the letter c where c~300,000,000 meters/second or 3x108 meters/second. Using f for frequency and l for wavelength, in vacuum the wavelength and frequency are related to the speed of light as fl=cthe relation between the speed of light, frequency, and wavelength in a vacuum.
3x108 m/s Vacuum is used as the reference for all conversions below. One must keep in mind that if the wave is propagating in a medium other than vacuum, the wave propagates at a speed slower than the speed of light.
IEEE Frequency Bands :
The lowest frequency of visible light (and thus energy), goes like this :
Red, orange, yellow, blue, indigo andviolet starting with the shortest wavelength.
Thus the highest energy:
Violet, indigo, blue, yellow, orange and red. |